Corticosteroids for treatment of COVID-19: effect, evidence, expectation and extent
- PMID: 34751250
- PMCID: PMC8567120
- DOI: 10.1186/s43088-021-00165-0
Corticosteroids for treatment of COVID-19: effect, evidence, expectation and extent
Abstract
Background: The World Health Organization (WHO) announced the COVID-19 occurrence as a global pandemic in March 2020. The treatment of SARS-CoV-2 patients is based on the experience gained from SARS-CoV and MERS-CoV infection during 2003. There is no clinically accepted therapeutic drug(s) accessible yet for the treatment of COVID-19.
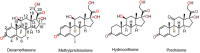
Main body: Corticosteroids, i.e., dexamethasone, methylprednisolone, hydrocortisone and prednisone are used alone or in combination for the treatment of moderate, severe and critically infected COVID-19 patients who are hospitalized and require supplemental oxygen as per current management strategies and guidelines for COVID-19 published by the National Institutes of Health. Corticosteroids are recorded in the WHO model list of essential medicines and are easily accessible worldwide at a cheaper cost in multiple formulations and various dosage forms. Corticosteroid can be used in all age group of patients, i.e., children, adult, elderly and during pregnancy or breastfeeding women. Corticosteroids have potent anti-inflammatory and immunosuppressive effects in both primary and secondary immune cells, thereby reducing the generation of proinflammatory cytokines and chemokines and lowering the activation of T cells, monocytes and macrophages. The corticosteroids should not be used in the treatment of non-severe COVID-19 patients because corticosteroids suppress the immune response and reduce the symptoms and associated side effects such as slow recovery, bacterial infections, hypokalemia, mucormycosis and finally increase the chances of death.
Conclusion: Intensive research on corticosteroid therapy in COVID-19 treatment is urgently needed to elucidate their mechanisms and importance in contributing toward successful prevention and treatment approaches. Hence, this review emphasizes on recent advancement on corticosteroid therapy for defining their importance in overcoming SARS-CoV-2 pandemic, their mechanism, efficacy and extent of corticosteroids in the treatment of COVID-19 patients.
Keywords: COVID-19; Corticosteroids; Dexamethasone; Hydrocortisone; Methylprednisolone; Prednisone.
© The Author(s) 2021.
Conflict of interest statement
Competing interestsThe authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials disclosed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Figures
References
-
- WHO Organization. Coronavirus disease 2019 (COVID-19): situation report, 72. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (2020).
-
- Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, Lagolio E, Celotto S, Pizzol D, Zou L, Tully MA, Ilie PC, Trott M, López-Sánchez GF, Smith L. Use of corticosteroids in Coronavirus Disease 2019 Pneumonia: a systematic review of the literature. Front Med. 2020;7:170. doi: 10.3389/fmed.2020.00170. - DOI - PMC - PubMed
-
- Chan JF-W, Yuan S, Kok K-H, To KK-W, Chu H, Yang J, Xing F, Liu J, Yip CC-Y, Poon RW-S. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous