Antibody response against SARS-CoV-2 in convalescent plasma donors: Can we predict subjects' eligibility?
- PMID: 34751255
- PMCID: PMC8566374
- DOI: 10.1016/j.htct.2021.07.008
Antibody response against SARS-CoV-2 in convalescent plasma donors: Can we predict subjects' eligibility?
Abstract
Introduction: As the Coronavirus Disease 2019 (COVID-19) pandemic unfolds around the world; answers related to the antibody response against the virus are necessary to develop treatment and prophylactic strategies. We attempted to understand part of the immune response of convalescent plasma donation candidates.
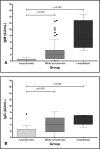
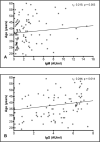
Method: We carried out a cross-sectional, observational, non-intervention study, testing 102 convalescent plasma donation candidates for antibodies against the virus, relating these data to the time interval between symptom onset and sample collection, age, disease severity, and gender.
Results: In our sample, the individuals who developed a greater antibody response were the ones who had a longer time interval between symptom onset and sample collection, the ones who had been hospitalized and the subjects above 35 years old. Moreover, 17 individuals did not present any reactive antibodies.
Conclusion: These results are important in that they raise questions about the role of the humoral response against the virus, as some individuals do not develop antibodies to fight it. In addition, they help develop recruitment strategies for convalescent plasma donors, who should be asymptomatic for at least 21 days and are possibly more likely to have reactive antibodies after 35 days without symptoms.
Keywords: Blood donation; COVID-19 convalescent plasma treatment; COVID-19 serological testing; Coronavirus infections.
© 2021 Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. Published by Elsevier España, S.L.U.
Conflict of interest statement
None.
Figures
References
-
- WHO . 2020. WHO Director-General's Opening Remarks at the Media Briefing on COVID-19 - 11 [Internet] [cited 2020 Sep 15]. Available from: https://www.who.int/director-general/speeches/detail/who-director-genera....
-
- Johns Hopkins . 2020. Center for System Science and Engineering. COVID-19 Map.https://coronavirus.jhu.edu/map.html [Internet][cited 2021 Jun 07]. Available from.
-
- Brasil . 2020. Secretaria de Vigilância à Saúde (SVS): Guia de vigilância Epidemiológica [Internet]https://covid.saude.gov.br [cited 2021 Jun 07]. Available from.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous