Quantifying Anticholinergic Burden and Sedative Load in Older Adults with Polypharmacy: A Systematic Review of Risk Scales and Models
- PMID: 34751922
- PMCID: PMC8592980
- DOI: 10.1007/s40266-021-00895-x
Quantifying Anticholinergic Burden and Sedative Load in Older Adults with Polypharmacy: A Systematic Review of Risk Scales and Models
Abstract
Background: Patients taking medication with high anticholinergic and sedative properties are at increased risk of experiencing poor cognitive and physical outcomes. Therefore, precise quantification of the cumulative burden of their drug regimen is advisable. There is no agreement regarding which scale to use to simultaneously quantify the burden associated with medications.
Objectives: The objective of this review was to assess the strengths and limitations of available tools to quantify medication-related anticholinergic burden and sedative load in older adults. We discuss specific limitations and agreements between currently available scales and models and propose a comprehensive table combining drugs categorized as high, moderate, low, or no anticholinergic or sedative activity as excerpted from the selected studies.
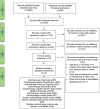
Methods: A targeted search was carried out using the National Library of Medicine through PubMed using medical subject heading terms and text words around the following search terms: (anticholinergic OR sedative) AND (load OR burden OR scale) for studies published between 1 January 1945 and 5 June 2021. In addition, the following databases were searched using the same terms: MEDLINE-EBSCO, APA PsycInfo, CINAHL Plus, Cochrane Library, Scopus, OAIster, OVID-MEDLINE, Web of Science, and Google Scholar. Screening by titles was followed by an abstract and full-text review. After blind evaluation, agreement between reviewers was reached to establish drug characteristics and categories.
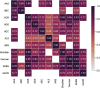
Results: After 3163 articles were identified, 13 were included: 11 assigned risk scores to anticholinergic drugs and two to sedative drugs. Considerable variability between anticholinergic scales was observed; scales included between 27 and 548 drugs. We generated a comprehensive table combining the anticholinergic and sedative activities of drugs evaluated and proposed a categorization of these drugs based on available scientific and clinical evidence. Our table combines information about 642 drugs and categorizes 44, 25, 99, and 474 drugs as high, moderate, low, or no anticholinergic and sedative activity, respectively.
Conclusions: Variability and inconsistency exists among scales used to categorize drugs with anticholinergic or sedative burden. In this review, we provide a comprehensive table that proposes a new categorization of these drugs. A longitudinal study will be required to validate the new proposed anticholinergic and sedative burden catalog in an evidence-based manner.
© 2021. The Author(s).
Conflict of interest statement
Sweilem B Al Rihani, Malavika Deodhar, Lucy I Darakjian, Pamela Dow, Matt K Smith, Ravil Bikmetov, Jacques Turgeon, and Veronique Michaud are employees and shareholders of Tabula Rasa HealthCare.
Figures

References
-
- Halli-Tierney AD, Scarbrough C, Carroll D. Polypharmacy: evaluating risks and deprescribing. Am Fam Physician. 2019;100(1):32–38. - PubMed
-
- Hales CM SJ, Martin CB, Kohen D. Prescription drug use among adults aged 40–79 in the United States and Canada. NCHS Data Brief No 347. 2019. https://www.cdc.gov/nchs/products/databriefs/db347.htm#::text=Nearly%207.... - PubMed
-
- Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5(4):345–351. - PubMed
-
- Barclay K, Frasetto A, Robb J, Mandel ED. Polypharmacy in the elderly: how to reduce adverse drug events. Clin Rev. 2018;28(2):38–44.
-
- Turgeon J, Michaud V, Steffen L. The dangers of polypharmacy in elderly patients. JAMA Intern Med. 2017;177(10):1544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

