Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age
- PMID: 34752019
- PMCID: PMC8609605
- DOI: 10.1056/NEJMoa2116298
Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age
Abstract
Background: Safe, effective vaccines against coronavirus disease 2019 (Covid-19) are urgently needed in children younger than 12 years of age.
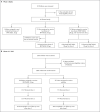
Methods: A phase 1, dose-finding study and an ongoing phase 2-3 randomized trial are being conducted to investigate the safety, immunogenicity, and efficacy of two doses of the BNT162b2 vaccine administered 21 days apart in children 6 months to 11 years of age. We present results for 5-to-11-year-old children. In the phase 2-3 trial, participants were randomly assigned in a 2:1 ratio to receive two doses of either the BNT162b2 vaccine at the dose level identified during the open-label phase 1 study or placebo. Immune responses 1 month after the second dose of BNT162b2 were immunologically bridged to those in 16-to-25-year-olds from the pivotal trial of two 30-μg doses of BNT162b2. Vaccine efficacy against Covid-19 at 7 days or more after the second dose was assessed.
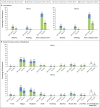
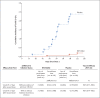
Results: During the phase 1 study, a total of 48 children 5 to 11 years of age received 10 μg, 20 μg, or 30 μg of the BNT162b2 vaccine (16 children at each dose level). On the basis of reactogenicity and immunogenicity, a dose level of 10 μg was selected for further study. In the phase 2-3 trial, a total of 2268 children were randomly assigned to receive the BNT162b2 vaccine (1517 children) or placebo (751 children). At data cutoff, the median follow-up was 2.3 months. In the 5-to-11-year-olds, as in other age groups, the BNT162b2 vaccine had a favorable safety profile. No vaccine-related serious adverse events were noted. One month after the second dose, the geometric mean ratio of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing titers in 5-to-11-year-olds to those in 16-to-25-year-olds was 1.04 (95% confidence interval [CI], 0.93 to 1.18), a ratio meeting the prespecified immunogenicity success criterion (lower bound of two-sided 95% CI, >0.67; geometric mean ratio point estimate, ≥0.8). Covid-19 with onset 7 days or more after the second dose was reported in three recipients of the BNT162b2 vaccine and in 16 placebo recipients (vaccine efficacy, 90.7%; 95% CI, 67.7 to 98.3).
Conclusions: A Covid-19 vaccination regimen consisting of two 10-μg doses of BNT162b2 administered 21 days apart was found to be safe, immunogenic, and efficacious in children 5 to 11 years of age. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04816643.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age.N Engl J Med. 2022 Feb 10;386(6):604. doi: 10.1056/NEJMc2118775. Epub 2022 Jan 19. N Engl J Med. 2022. PMID: 35045223 No abstract available.
-
BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age.N Engl J Med. 2022 Feb 10;386(6):604-606. doi: 10.1056/NEJMc2118775. Epub 2022 Jan 19. N Engl J Med. 2022. PMID: 35045224 No abstract available.
-
More on BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age.N Engl J Med. 2022 Mar 24;386(12):1191-1192. doi: 10.1056/NEJMc2201556. Epub 2022 Mar 2. N Engl J Med. 2022. PMID: 35235722 No abstract available.
-
More on BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age.N Engl J Med. 2022 Mar 24;386(12):1192. doi: 10.1056/NEJMc2201556. Epub 2022 Mar 2. N Engl J Med. 2022. PMID: 35235723 No abstract available.
-
Four points regarding reproducibility and external statistical validity.J Evid Based Med. 2022 Dec;15(4):317-319. doi: 10.1111/jebm.12498. Epub 2022 Oct 17. J Evid Based Med. 2022. PMID: 36253959 Free PMC article. No abstract available.
References
-
- Sahin U, Muik A, Vogler I, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021;595:572-577. - PubMed
-
- Comirnaty and Pfizer–BioNTech COVID-19 Vaccine. Food and Drug Administration, Silver Spring, MD, October 29, 2021. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise...).
-
- Comirnaty (Covid-19 vaccine, mRNA) prescribing information. Mainz, Germany: BioNTech Manufacturing, 2021. (https://www.fda.gov/media/151707/download).
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
