High-level synthesis and secretion of laccase, a metalloenzyme biocatalyst, by the halophilic archaeon Haloferax volcanii
- PMID: 34752290
- PMCID: PMC8867731
- DOI: 10.1016/bs.mie.2021.05.012
High-level synthesis and secretion of laccase, a metalloenzyme biocatalyst, by the halophilic archaeon Haloferax volcanii
Abstract
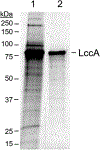
Haloarchaea and their enzymes have extremophilic properties desirable for use as platform organisms and biocatalysts in the bioindustry. These GRAS (generally regarded as safe) designated microbes thrive in hypersaline environments and use a salt-in strategy to maintain osmotic homeostasis. This unusual strategy has resulted in the evolution of most of the intracellular and extracellular enzymes of haloarchaea to be active and stable not only in high salt (2-5M) but also in low salt (0.2M). This salt tolerance is correlated with a resilience to low water activity, thus, rendering the haloarchaeal enzymes active and stable in organic solvent and temperatures of 50-60°C used in the enzymatic biodelignification and saccharification of lignocellulosic materials. High-level secretion of haloarchaeal enzymes to the extracellular milieu is useful for many applications, including enzymes that deconstruct biomass to allow for lignin depolymerization and simultaneous fermentation of sugars released from hemicellulose and cellulose fractions of lignocellulosics. Here we detail strategies and methods useful for high-level secretion of a laccase, HvLccA, that mediates oxidation of various phenolics by engineering a recombinant strain of the haloarchaeon Haloferax volcanii.
Keywords: Archaea; Halophilic; Laccase; Metalloenzyme; Organic solvent-tolerant; Secretion.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

References
-
- Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, et al. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology, 37(4), 420–423. - PubMed
-
- Blanch HW, Simmons BA, & Klein-Marcuschamer D (2011). Biomass deconstruction to sugars. Biotechnology Journal, 6(9), 1086–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

