Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease
- PMID: 34752422
- PMCID: PMC8718145
- DOI: 10.1172/JCI142260
Tryptophan metabolites suppress the Wnt pathway and promote adverse limb events in chronic kidney disease
Abstract
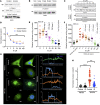
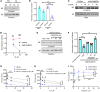
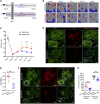
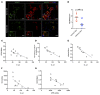
Chronic kidney disease (CKD) imposes a strong and independent risk for peripheral artery disease (PAD). While solutes retained in CKD patients (uremic solutes) inflict vascular damage, their role in PAD remains elusive. Here, we show that the dietary tryptophan-derived uremic solutes including indoxyl sulfate (IS) and kynurenine (Kyn) at concentrations corresponding to those in CKD patients suppress β-catenin in several cell types, including microvascular endothelial cells (ECs), inhibiting Wnt activity and proangiogenic Wnt targets in ECs. Mechanistic probing revealed that these uremic solutes downregulated β-catenin in a manner dependent on serine 33 in its degron motif and through the aryl hydrocarbon receptor (AHR). Hindlimb ischemia in adenine-induced CKD and IS solute-specific mouse models showed diminished β-catenin and VEGF-A in the capillaries and reduced capillary density, which correlated inversely with blood levels of IS and Kyn and AHR activity in ECs. An AHR inhibitor treatment normalized postischemic angiogenic response in CKD mice to a non-CKD level. In a prospective cohort of PAD patients, plasma levels of tryptophan metabolites and plasma's AHR-inducing activity in ECs significantly increased the risk of future adverse limb events. This work uncovers the tryptophan metabolite/AHR/β-catenin axis as a mediator of microvascular rarefaction in CKD patients and demonstrates its targetability for PAD in CKD models.
Keywords: Chronic kidney disease; Nephrology; Vascular Biology.
Figures






Comment in
-
Indoxyl sulfate in uremia: an old idea with updated concepts.J Clin Invest. 2022 Jan 4;132(1):e155860. doi: 10.1172/JCI155860. J Clin Invest. 2022. PMID: 34981787 Free PMC article.

