Small molecule allosteric inhibitors of RORγt block Th17-dependent inflammation and associated gene expression in vivo
- PMID: 34752458
- PMCID: PMC8577775
- DOI: 10.1371/journal.pone.0248034
Small molecule allosteric inhibitors of RORγt block Th17-dependent inflammation and associated gene expression in vivo
Abstract
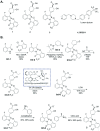
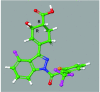
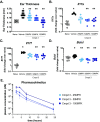
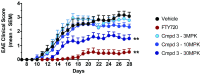
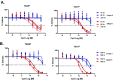
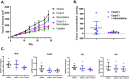
Retinoic acid receptor-related orphan nuclear receptor (ROR) γt is a member of the RORC nuclear hormone receptor family of transcription factors. RORγt functions as a critical regulator of thymopoiesis and immune responses. RORγt is expressed in multiple immune cell populations including Th17 cells, where its primary function is regulation of immune responses to bacteria and fungi through IL-17A production. However, excessive IL-17A production has been linked to numerous autoimmune diseases. Moreover, Th17 cells have been shown to elicit both pro- and anti-tumor effects. Thus, modulation of the RORγt/IL-17A axis may represent an attractive therapeutic target for the treatment of autoimmune disorders and some cancers. Herein we report the design, synthesis and characterization of three selective allosteric RORγt inhibitors in preclinical models of inflammation and tumor growth. We demonstrate that these compounds can inhibit Th17 differentiation and maintenance in vitro and Th17-dependent inflammation and associated gene expression in vivo, in a dose-dependent manner. Finally, RORγt inhibitors were assessed for efficacy against tumor formation. While, RORγt inhibitors were shown to inhibit tumor formation in pancreatic ductal adenocarcinoma (PDAC) organoids in vitro and modulate RORγt target genes in vivo, this activity was not sufficient to delay tumor volume in a KP/C human tumor mouse model of pancreatic cancer.
Conflict of interest statement
All authors are either present or former employees of Celgene Corporation and/or Bristol Myers Squibb and own stocks and/or shares of Bristol Myers Squibb. All work for the current study was performed by authors only while employed at Celgene Corporation or Bristol Myers Squibb. Celgene Corporation is a wholly owned subsidiary of Bristol Myers Squibb. The Bristol Myers Squibb was the sole funder for this study and provided support in the form of salaries for all authors, but did not have any additional role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. Jenna Malley & Lawrence G. Hamann are currently employed by Takeda Pharmaceuticals. John Malona is currently employed by Jnana Therapeutics. C. Eric Schwartz is currently employed by Cedilla Therapeutics. Meghan Clements is currently employed by AbbVie. Ganesh Rajaraman is currently employed by Novartis Pharmaceuticals. John Cho is currently employed by KSQ Therapeutics. Neither Takeda Pharmaceuticals, Jnana Therapeutics, Cedilla Therapeutics, AbbVie, Novartis Pharmaceuticals or KSQ Therapeutics provided funding to the current work and these affiliations do not alter the authors’ adherence to PLOS ONE policies on sharing data and materials.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

