Impact of extrinsic incubation temperature on natural selection during Zika virus infection of Aedes aegypti and Aedes albopictus
- PMID: 34752502
- PMCID: PMC8629396
- DOI: 10.1371/journal.ppat.1009433
Impact of extrinsic incubation temperature on natural selection during Zika virus infection of Aedes aegypti and Aedes albopictus
Abstract
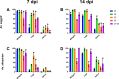
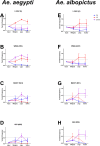
Arthropod-borne viruses (arboviruses) require replication across a wide range of temperatures to perpetuate. While vertebrate hosts tend to maintain temperatures of approximately 37°C-40°C, arthropods are subject to ambient temperatures which can have a daily fluctuation of > 10°C. Temperatures impact vector competence, extrinsic incubation period, and mosquito survival unimodally, with optimal conditions occurring at some intermediate temperature. In addition, the mean and range of daily temperature fluctuations influence arbovirus perpetuation and vector competence. The impact of temperature on arbovirus genetic diversity during systemic mosquito infection, however, is poorly understood. Therefore, we determined how constant extrinsic incubation temperatures of 25°C, 28°C, 32°C, and 35°C control Zika virus (ZIKV) vector competence and population dynamics within Aedes aegypti and Aedes albopictus mosquitoes. We also examined fluctuating temperatures which better mimic field conditions in the tropics. We found that vector competence varied in a unimodal manner for constant temperatures peaking between 28°C and 32°C for both Aedes species. Transmission peaked at 10 days post-infection for Aedes aegypti and 14 days for Aedes albopictus. Conversely, fluctuating temperature decreased vector competence. Using RNA-seq to characterize ZIKV population structure, we identified that temperature alters the selective environment in unexpected ways. During mosquito infection, constant temperatures more often elicited positive selection whereas fluctuating temperatures led to strong purifying selection in both Aedes species. These findings demonstrate that temperature has multiple impacts on ZIKV biology, including major effects on the selective environment within mosquitoes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Meyer A, Freier M, Schmidt T, Rostowski K, Zwoch J, Lilie H, et al. An RNA Thermometer Activity of the West Nile Virus Genomic 3’-Terminal Stem-Loop Element Modulates Viral Replication Efficiency during Host Switching. Viruses. 2020;12(1). Epub 2020/01/19. doi: 10.3390/v12010104 ; PubMed Central PMCID: PMC7019923. - DOI - PMC - PubMed
-
- Grubaugh ND, Weger-Lucarelli J, Murrieta RA, Fauver JR, Garcia-Luna SM, Prasad AN, et al. Genetic Drift during Systemic Arbovirus Infection of Mosquito Vectors Leads to Decreased Relative Fitness during Host Switching. Cell Host Microbe. 2016;19(4):481–92. Epub 2016/04/07. doi: 10.1016/j.chom.2016.03.002 ; PubMed Central PMCID: PMC4833525. - DOI - PMC - PubMed
-
- Garmel GM. An Introduction to Clinical Emergency Medicine. 2nd ed 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

