Maintenance and loss of endocytic organelle integrity: mechanisms and implications for antigen cross-presentation
- PMID: 34753318
- PMCID: PMC8580422
- DOI: 10.1098/rsob.210194
Maintenance and loss of endocytic organelle integrity: mechanisms and implications for antigen cross-presentation
Abstract
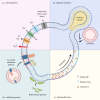
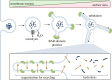
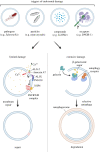
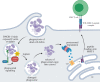
The membranes of endosomes, phagosomes and macropinosomes can become damaged by the physical properties of internalized cargo, by active pathogenic invasion or by cellular processes, including endocytic maturation. Loss of membrane integrity is often deleterious and is, therefore, prevented by mitigation and repair mechanisms. However, it can occasionally be beneficial and actively induced by cells. Here, we summarize the mechanisms by which cells, in particular phagocytes, try to prevent membrane damage and how, when this fails, they repair or destroy damaged endocytic organelles. We also detail how one type of phagocyte, the dendritic cell, can deliberately trigger localized damage to endocytic organelles to allow for major histocompatibility complex class I presentation of exogenous antigens and initiation of CD8+ T-cell responses to viruses and tumours. Our review highlights mechanisms for the regulation of endocytic organelle membrane integrity at the intersection of cell biology and immunology that could be co-opted for improving vaccination and intracellular drug delivery.
Keywords: cross-presentation; endosomal escape; endosome; membrane damage; membrane repair; phagosome.
Conflict of interest statement
C.R.S. is a founder and stockholder of Adendra Therapeutics and owns stock options and/or is a paid consultant for Adendra Therapeutics, Bicara Therapeutics, Montis Biosciences, Oncurious NV, Bicycle Therapeutics and Sosei Heptares. C.R.S. holds a professorship at Imperial College London and honorary professorships at University College London and King's College London. None of these activities is related to this work.
Figures
References
-
- Lewis WH. 1937. Pinocytosis by malignant cells. Am. J. Cancer Res. 29, 666-679. (10.1158/ajc.1937.510) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
