Preferential and persistent impact of acute HIV-1 infection on CD4+ iNKT cells in colonic mucosa
- PMID: 34753817
- PMCID: PMC8609642
- DOI: 10.1073/pnas.2104721118
Preferential and persistent impact of acute HIV-1 infection on CD4+ iNKT cells in colonic mucosa
Abstract
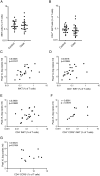
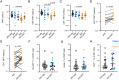
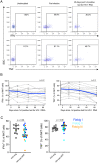
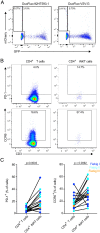
Acute HIV-1 infection (AHI) results in the widespread depletion of CD4+ T cells in peripheral blood and gut mucosal tissue. However, the impact on the predominantly CD4+ immunoregulatory invariant natural killer T (iNKT) cells during AHI remains unknown. Here, iNKT cells from peripheral blood and colonic mucosa were investigated during treated and untreated AHI. iNKT cells in blood were activated and rapidly depleted in untreated AHI. At the time of peak HIV-1 viral load, these cells showed the elevated expression of cell death-associated transcripts compared to preinfection. Residual peripheral iNKT cells suffered a diminished responsiveness to in vitro stimulation early into chronic infection. Additionally, HIV-1 DNA, as well as spliced and unspliced viral RNA, were detected in iNKT cells isolated from blood, indicating the active infection of these cells in vivo. The loss of iNKT cells occurred from Fiebig stage III in the colonic mucosa, and these cells were not restored to normal levels after initiation of ART during AHI. CD4+ iNKT cells were depleted faster and more profoundly than conventional CD4+ T cells, and the preferential infection of CD4+ iNKT cells over conventional CD4+ T cells was confirmed by in vitro infection experiments. In vitro data also provided evidence of latent infection in iNKT cells. Strikingly, preinfection levels of peripheral blood CD4+ iNKT cells correlated directly with the peak HIV-1 load. These findings support a model in which iNKT cells are early targets for HIV-1 infection, driving their rapid loss from circulation and colonic mucosa.
Keywords: ART; HIV-1; gut; iNKT cells; immune activation.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: J.A. previously received honoraria from Merck, ViiV Healthcare, Roche, AbbVie, and Gilead for her participation in advisory meetings.
Figures



References
-
- Brennan P. J., Brigl M., Brenner M. B., Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat. Rev. Immunol. 13, 101–117 (2013). - PubMed
-
- Cohen N. R., Garg S., Brenner M. B., Antigen presentation by CD1 lipids, T cells, and NKT cells in microbial immunity. Adv. Immunol. 102, 1–94 (2009). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

