The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant
- PMID: 34753894
- PMCID: PMC8942603
- DOI: 10.1097/TP.0000000000003983
The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant
Abstract
Background: In kidney patients COVID-19 is associated with severely increased morbidity and mortality. A comprehensive comparison of the immunogenicity, tolerability, and safety of COVID-19 vaccination in different cohorts of kidney patients and a control cohort is lacking.
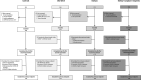
Methods: This investigator driven, prospective, controlled multicenter study included 162 participants with chronic kidney disease (CKD) stages G4/5 (eGFR < 30 mL/min/1.73m2), 159 participants on dialysis, 288 kidney transplant recipients, and 191 controls. Participants received 2 doses of the mRNA-1273 COVID-19 vaccine (Moderna). The primary endpoint was seroconversion.
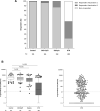
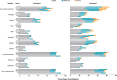
Results: Transplant recipients had a significantly lower seroconversion rate when compared with controls (56.9% versus 100%, P < 0.001), with especially mycophenolic acid, but also, higher age, lower lymphocyte concentration, lower eGFR, and shorter time after transplantation being associated with nonresponder state. Transplant recipients also showed significantly lower titers of neutralizing antibodies and T-cell responses when compared with controls. Although a high seroconversion rate was observed for participants with CKD G4/5 (100%) and on dialysis (99.4%), mean antibody concentrations in the CKD G4/5 cohort and dialysis cohort were lower than in controls (2405 [interquartile interval 1287-4524] and 1650 [698-3024] versus 3186 [1896-4911] BAU/mL, P = 0.06 and P < 0.001, respectively). Dialysis patients and especially kidney transplant recipients experienced less systemic vaccination related adverse events. No specific safety issues were noted.
Conclusions: The immune response following vaccination in patients with CKD G4/5 and on dialysis is almost comparable to controls. In contrast, kidney transplant recipients have a poor response. In this latter, patient group development of alternative vaccination strategies are warranted.
Trial registration: ClinicalTrials.gov NCT04741386.
Copyright © 2021 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest
Figures




References
-
- Reddy S, Chitturi C, Yee J. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26:72–78. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

