Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway
- PMID: 34753919
- PMCID: PMC8578653
- DOI: 10.1038/s41419-021-04363-7
Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and repress apoptosis in stroke mice via the TGF-β/Smad2/3 pathway
Abstract
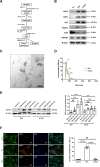
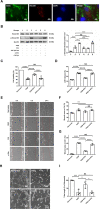
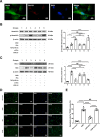
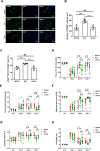
Systemic transplantation of oxygen-glucose deprivation (OGD)-preconditioned primary microglia enhances neurological recovery in rodent stroke models, albeit the underlying mechanisms have not been sufficiently addressed. Herein, we analyzed whether or not extracellular vesicles (EVs) derived from such microglia are the biological mediators of these observations and which signaling pathways are involved in the process. Exposing bEnd.3 endothelial cells (ECs) and primary cortical neurons to OGD, the impact of EVs from OGD-preconditioned microglia on angiogenesis and neuronal apoptosis by the tube formation assay and TUNEL staining was assessed. Under these conditions, EV treatment stimulated both angiogenesis and tube formation in ECs and repressed neuronal cell injury. Characterizing microglia EVs by means of Western blot analysis and other techniques revealed these EVs to be rich in TGF-β1. The latter turned out to be a key compound for the therapeutic potential of microglia EVs, affecting the Smad2/3 pathway in both ECs and neurons. EV infusion in stroke mice confirmed the aforementioned in vitro results, demonstrating an activation of the TGF-β/Smad2/3 signaling pathway within the ischemic brain. Furthermore, enriched TGF-β1 in EVs secreted from OGD-preconditioned microglia stimulated M2 polarization of residing microglia within the ischemic cerebral environment, which may contribute to a regulation of an early inflammatory response in postischemic hemispheres. These observations are not only interesting from the mechanistic point of view but have an immediate therapeutic implication as well, since stroke mice treated with such EVs displayed a better functional recovery in the behavioral test analyses. Hence, the present findings suggest a new way of action of EVs derived from OGD-preconditioned microglia by regulating the TGF-β/Smad2/3 pathway in order to promote tissue regeneration and neurological recovery in stroke mice.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. - PubMed
-
- Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–66. - PubMed
-
- De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. 2016;47:1165–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

