Tunable, biodegradable grafting-from glycopolypeptide bottlebrush polymers
- PMID: 34753949
- PMCID: PMC8578664
- DOI: 10.1038/s41467-021-26808-5
Tunable, biodegradable grafting-from glycopolypeptide bottlebrush polymers
Abstract
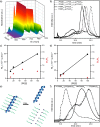
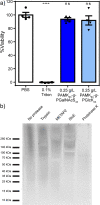
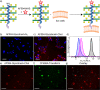
The cellular glycocalyx and extracellular matrix are rich in glycoproteins and proteoglycans that play essential physical and biochemical roles in all life. Synthetic mimics of these natural bottlebrush polymers have wide applications in biomedicine, yet preparation has been challenged by their high grafting and glycosylation densities. Using one-pot dual-catalysis polymerization of glycan-bearing α-amino acid N-carboxyanhydrides, we report grafting-from glycopolypeptide brushes. The materials are chemically and conformationally tunable where backbone and sidechain lengths were precisely altered, grafting density modulated up to 100%, and glycan density and identity tuned by monomer feed ratios. The glycobrushes are composed entirely of sugars and amino acids, are non-toxic to cells, and are degradable by natural proteases. Inspired by native lipid-anchored proteoglycans, cholesterol-modified glycobrushes were displayed on the surface of live human cells. Our materials overcome long-standing challenges in glycobrush polymer synthesis and offer new opportunities to examine glycan presentation and multivalency from chemically defined scaffolds.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Varki, A. & Sharon, N. Essentials of Glycobiology, 2nd edn. 10.1016/S0962-8924(00)01855-9 (Cold Spring Harbor Laboratory Press, 2009). - PubMed
-
- Lindahl, U., Couchman, J., Kimata, K. & Esko, J. D. Proteoglycans and Sulfated Glycosaminoglycans. Essentials of Glycobiology (Cold Spring Harbor Laboratory Press, 2015). - PubMed
-
- Bohley P. Biochemical nomenclature and related documents. FEBS Lett. 1980;116:135–135. doi: 10.1016/0014-5793(80)80559-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

