New and simplified method for drug combination studies by checkerboard assay
- PMID: 34754811
- PMCID: PMC8563647
- DOI: 10.1016/j.mex.2021.101543
New and simplified method for drug combination studies by checkerboard assay
Abstract
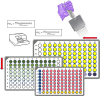
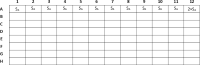
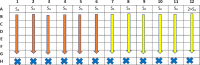
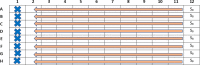
To evaluate the effect of two combined antimicrobial drugs, one method currently in use is the checkerboard assay in a 96-well microplate, which gives a good in vivo estimation of the drug-drug combination effect. Appropriate and consolidated methods are described in numerous scientific publications which are, however, in turn, laborious and time-spending, specifically for the setting of the 96-well microplate preparation. Each drug of every combination must be prepared and dispensed individually in several steps, often limiting its use in terms of consumed materials and working time. In our method, the strengths of the previous consolidated techniques are kept, although the toughness and the execution time are drastically reduced. No special laboratory apparatuses are needed. All the procedures of our method can be referred to the CLSI or EUCAST guideline. The method provides few main steps, which can be summarised in:•Preparation of the microorganism inoculum and three concentrations of antimicrobial drugs.•Easy dispensing of all reagents into the microplates with a multichannel pipette.•Evaluation of the microorganism optical density (OD) by a microplate reader, and calculation of growth percentage for each of the 77 combinations.
Keywords: Antagonism; Antimicrobials; CLSI; Drug-drug interaction; EUCAST; MIC; Minimal inhibitory concentration; Synergy.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Garcia L. Clinical Microbiology Procedures Handbook. 3rd ed. American Society of Microbiology; 2014. Synergism testing: broth microdilution checkerboard and broth macrodilution methods; pp. 140–162. - DOI
-
- Wayne PA. CLSI supplement M100. 30th ed. Clinical and Laboratory Standards Institute; 2020. CLSI.Standards for Antimicrobial Susceptibility Testing.
-
- "The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 10.0, 2020"
-
- Segatore B., Bellio P., Setacci D., Brisdelli F., Piovano M., Garbarino J.A., Nicoletti M., Amicosante G., Perilli M., Celenza G. In vitro interaction of usnic acid in combination with antimicrobial agents against methicillin-resistant Staphylococcus aureus clinical isolates determined by FICI and Δe model methods. Phytomedicine. 2012;19:341–347. doi: 10.1016/j.phymed.2011.10.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources