A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation
- PMID: 34755092
- PMCID: PMC8564056
- DOI: 10.1016/j.isci.2021.103252
A novel jamming phase diagram links tumor invasion to non-equilibrium phase separation
Abstract
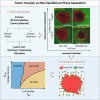
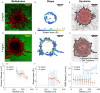
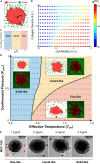
It is well established that the early malignant tumor invades surrounding extracellular matrix (ECM) in a manner that depends upon material properties of constituent cells, surrounding ECM, and their interactions. Recent studies have established the capacity of the invading tumor spheroids to evolve into coexistent solid-like, fluid-like, and gas-like phases. Using breast cancer cell lines invading into engineered ECM, here we show that the spheroid interior develops spatial and temporal heterogeneities in material phase which, depending upon cell type and matrix density, ultimately result in a variety of phase separation patterns at the invasive front. Using a computational approach, we further show that these patterns are captured by a novel jamming phase diagram. We suggest that non-equilibrium phase separation based upon jamming and unjamming transitions may provide a unifying physical picture to describe cellular migratory dynamics within, and invasion from, a tumor.
Keywords: Biophysics; Cancer; Mechanobiology.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

