Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men
- PMID: 34755128
- PMCID: PMC8561167
- DOI: 10.1016/j.xcrm.2021.100408
Altered brown fat thermoregulation and enhanced cold-induced thermogenesis in young, healthy, winter-swimming men
Abstract
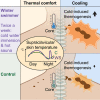
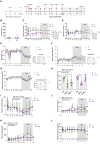
The Scandinavian winter-swimming culture combines brief dips in cold water with hot sauna sessions, with conceivable effects on body temperature. We study thermogenic brown adipose tissue (BAT) in experienced winter-swimming men performing this activity 2-3 times per week. Our data suggest a lower thermal comfort state in the winter swimmers compared with controls, with a lower core temperature and absence of BAT activity. In response to cold, we observe greater increases in cold-induced thermogenesis and supraclavicular skin temperature in the winter swimmers, whereas BAT glucose uptake and muscle activity increase similarly to those of the controls. All subjects demonstrate nocturnal reduction in supraclavicular skin temperature, whereas a distinct peak occurs at 4:30-5:30 a.m. in the winter swimmers. Our data leverage understanding of BAT in adult human thermoregulation, suggest both heat and cold acclimation in winter swimmers, and propose winter swimming as a potential strategy for increasing energy expenditure.
Trial registration: ClinicalTrials.gov NCT03096535.
Keywords: adipose tissue; cold acclimation; cold water immersion; core temperature; energy expenditure; heat acclimation; human brown fat; human circadian rhythm; sauna; thermal comfort.
© 2021 The Authors.
Conflict of interest statement
C. Scheele is a consultant for Novo Nordisk A/S on human brown adipose tissue biology. Z.G.-H. works, in some capacity, for Embark Biotech ApS, a company developing therapeutics for the treatment of diabetes and obesity. All other authors declare no competing interests associated with this manuscript.
Figures

Comment in
-
Perspectives on the role of brown adipose tissue in human body temperature and metabolism.Cell Rep Med. 2021 Oct 19;2(10):100427. doi: 10.1016/j.xcrm.2021.100427. eCollection 2021 Oct 19. Cell Rep Med. 2021. PMID: 34755138 Free PMC article.
References
-
- Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293:E444–E452. - PubMed
-
- Lee P., Bova R., Schofield L., Bryant W., Dieckmann W., Slattery A., Govendir M.A., Emmett L., Greenfield J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016;23:602–609. - PubMed
-
- van den Berg R., Kooijman S., Noordam R., Ramkisoensing A., Abreu-Vieira G., Tambyrajah L.L., Dijk W., Ruppert P., Mol I.M., Kramar B. A Diurnal Rhythm in Brown Adipose Tissue Causes Rapid Clearance and Combustion of Plasma Lipids at Wakening. Cell Rep. 2018;22:3521–3533. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

