The Landscape of COVID-19 Research in the United States: a Cross-sectional Study of Randomized Trials Registered on ClinicalTrials.Gov
- PMID: 34755268
- PMCID: PMC8577643
- DOI: 10.1007/s11606-021-07167-9
The Landscape of COVID-19 Research in the United States: a Cross-sectional Study of Randomized Trials Registered on ClinicalTrials.Gov
Abstract
Importance: SARS-CoV-2 has infected over 200 million people worldwide, resulting in more than 4 million deaths. Randomized controlled trials are the single best tool to identify effective treatments against this novel pathogen.
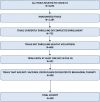
Objective: To describe the characteristics of randomized controlled trials of treatments for COVID-19 in the United States launched in the first 9 months of the pandemic. Design, Setting, and Participants We conducted a cross-sectional study of all completed or actively enrolling randomized, interventional, clinical trials for the treatment of COVID-19 in the United States registered on www.clinicaltrials.gov as of August 10, 2020. We excluded trials of vaccines and other interventions intended to prevent COVID-19. Main Outcomes and Measures We used descriptive statistics to characterize the clinical trials and the statistical power for the available studies. For the late-phase trials (i.e., phase 3 and 2/3 studies), we compared the geographic distribution of the clinical trials with the geographic distribution of people diagnosed with COVID-19.
Results: We identified 200 randomized controlled trials of treatments for people with COVID-19. Across all trials, 87 (43.5%) were single-center, 64 (32.0%) were unblinded, and 80 (40.0%) were sponsored by industry. The most common treatments included monoclonal antibodies (N=46 trials), small molecule immunomodulators (N=28), antiviral medications (N=24 trials), and hydroxychloroquine (N=20 trials). Of the 9 trials completed by August 2020, the median sample size was 450 (IQR 67-1113); of the 191 ongoing trials, the median planned sample size was 150 (IQR 60-400). Of the late-phase trials (N=54), the most common primary outcome was a severity scale (N=23, 42.6%), followed by a composite of mortality and ventilation (N=10, 18.5%), and mortality alone (N=6, 11.1%). Among these late-phase trials, all trials of antivirals, monoclonal antibodies, or chloroquine/hydroxychloroquine had a power of less than 25% to detect a 20% relative risk reduction in mortality. Had the individual trials for a given class of treatments instead formed a single trial, the power to detect that same reduction in mortality would have been greater than 98%. There was large variability in access to trials with the highest number of trials per capita in the Northeast and the lowest in the Midwest.
Conclusions and relevance: A large number of randomized trials were launched early in the pandemic to evaluate treatments for COVID-19. However, many trials were underpowered for important clinical endpoints and substantial geographic disparities were observed, highlighting the importance of improving national clinical trial infrastructure.
© 2021. Society of General Internal Medicine.
Conflict of interest statement
Drs. Sacks, North, Wolf, and Campbell report no conflicts of interest. Dr. Dougan has received consulting fees from Tillotts Pharma, Partner Therapeutics, ORIC pharmaceuticals, and Moderna; research funding from Novartis and Eli Lilly and is a member of the scientific advisory board of Neoleukin Therapeutics. Dr. Fralick is a consultant for ProofDx (previously Pine Trees Health), a start-up company developing a CRISPR-based diagnostic test for COVID-19.
Figures


Similar articles
-
Pharmacological Studies in Hospitalized COVID-19 Patients in Belgium: We Could Do Better.Viruses. 2022 Jun 29;14(7):1427. doi: 10.3390/v14071427. Viruses. 2022. PMID: 35891407 Free PMC article. Review.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Efficacy of hydroxychloroquine for post-exposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among adults exposed to coronavirus disease (COVID-19): a structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):475. doi: 10.1186/s13063-020-04446-4. Trials. 2020. PMID: 32493478 Free PMC article.
-
Controlled, double-blind, randomized trial to assess the efficacy and safety of hydroxychloroquine chemoprophylaxis in SARS CoV2 infection in healthcare personnel in the hospital setting: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Jun 3;21(1):472. doi: 10.1186/s13063-020-04400-4. Trials. 2020. PMID: 32493494 Free PMC article.
-
Clinical trial research agenda on COVID-19 - the first two years in Germany and beyond.Z Evid Fortbild Qual Gesundhwes. 2022 Nov;174:32-42. doi: 10.1016/j.zefq.2022.08.003. Epub 2022 Sep 28. Z Evid Fortbild Qual Gesundhwes. 2022. PMID: 36180342 Free PMC article. Review.
Cited by
-
Clinical trials and their impact on policy during COVID-19: a review.Wellcome Open Res. 2024 Jan 30;9:20. doi: 10.12688/wellcomeopenres.19305.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 38434720 Free PMC article. Review.
-
A bibliometric analysis of six decades of camel research in North Africa: Trends, collaboration, and emerging themes.Open Vet J. 2024 Dec;14(12):3505-3524. doi: 10.5455/OVJ.2024.v14.i12.34. Epub 2024 Dec 31. Open Vet J. 2024. PMID: 39927358 Free PMC article.
-
Pharmacological Studies in Hospitalized COVID-19 Patients in Belgium: We Could Do Better.Viruses. 2022 Jun 29;14(7):1427. doi: 10.3390/v14071427. Viruses. 2022. PMID: 35891407 Free PMC article. Review.
-
Do hospitals that participate in COVID-19 research differ from non-trial hospitals? A cross-sectional study of US hospitals.Trials. 2023 Aug 7;24(1):504. doi: 10.1186/s13063-023-07450-6. Trials. 2023. PMID: 37550662 Free PMC article.
References
-
- Andrew S. The US has 4% of the world’s population but 25% of its coronavirus cases. CNN. Published online June 2020. https://www.cnn.com/2020/06/30/health/us-coronavirus-toll-in-numbers-jun...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

