The Evaluation of Iron Deficiency and Iron Overload
- PMID: 34755596
- PMCID: PMC8941656
- DOI: 10.3238/arztebl.m2021.0290
The Evaluation of Iron Deficiency and Iron Overload
Abstract
Background: In the western world, 10-15% of women of child-bearing age suffer from iron-deficiency anemia. Iron overload due to chronic treatment with blood transfusions or hereditary hemochromatosis is much rarer.
Methods: This review is based on pertinent publications retrieved by a selective search on the pathophysiology, clinical features, and diagnostic evaluation of iron deficiency and iron overload.
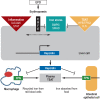
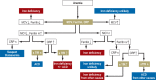
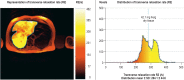
Results: The main causes of iron deficiency are malnutrition and blood loss. Its differential diagnosis includes iron-refractory iron deficiency anemia (IRIDA), a rare congenital disease in which the hepcidin level is pathologically elevated, as well as the more common anemia of chronic disease (anemia of chronic inflammation), in which increased amounts of hepcidin are formed under the influence of interleukin-6 and enteric iron uptake is blocked as a result. Iron overload comes about through long-term transfusion treatment or a congenital disturbance of iron metabolism (hemochromatosis). Its diagnostic evaluation is based on clinical and laboratory findings, imaging studies, and specific mutation analyses.
Conclusion: Our improving understanding of the molecular pathophysiology of iron metabolism aids in the evaluation of iron deficiency and iron overload and may in future enable treatment not just with iron supplementation or iron chelation, but also with targeted pharmacological modulation of the hepcidin regulatory system.
Figures
Comment in
-
Reference Ranges Should Be Updated.Dtsch Arztebl Int. 2022 Jun 17;119(24):427. doi: 10.3238/arztebl.m2022.0136. Dtsch Arztebl Int. 2022. PMID: 36106877 Free PMC article. No abstract available.
-
Iron Balance in Chronic Kidney Disease.Dtsch Arztebl Int. 2022 Jun 17;119(24):428. doi: 10.3238/arztebl.m2022.0138. Dtsch Arztebl Int. 2022. PMID: 36106879 Free PMC article. No abstract available.
-
In Reply.Dtsch Arztebl Int. 2022 Jun 17;119(24):428. doi: 10.3238/arztebl.m2022.0139. Dtsch Arztebl Int. 2022. PMID: 36106880 Free PMC article. No abstract available.
References
-
- Muckenthaler M, Petrides PE. Heinrich PC, Müller M, Graeve L, Koch HG, editors. Spurenelemente. Biochemie und Pathobiochemie Heidelberg: Springer Verlag. 2021:1042–1069.
-
- Corradini E, Buzzetti E, Pietrangelo A. Genetic iron overload disorders. Mol Aspects Med. 2020;75 100896. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

