Sperm flagellar 2 (SPEF2) is essential for sperm flagellar assembly in humans
- PMID: 34755699
- PMCID: PMC9295471
- DOI: 10.4103/aja202154
Sperm flagellar 2 (SPEF2) is essential for sperm flagellar assembly in humans
Abstract
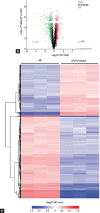
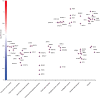
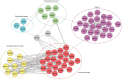
Spermiogenesis is a complex and tightly regulated process, consisting of acrosomal biogenesis, condensation of chromatin, flagellar assembly, and disposal of extra cytoplasm. Previous studies have reported that sperm flagellar 2 (SPEF2) deficiency causes severe asthenoteratozoospermia owing to spermiogenesis failure, but the underlying molecular mechanism in humans remains unclear. Here, we performed proteomic analysis on spermatozoa from three SPEF2 mutant patients to study the functional role of SPEF2 during sperm tail development. A total of 1262 differentially expressed proteins were detected, including 486 upregulated and 776 downregulated. The constructed heat map of the differentially expressed proteins showed similar trends. Among these, the expression of proteins related to flagellar assembly, including SPEF2, sperm associated antigen 6 (SPAG6), dynein light chain tctex-type 1 (DYNLT1), radial spoke head component 1 (RSPH1), translocase of outer mitochondrial membrane 20 (TOM20), EF-hand domain containing 1 (EFHC1), meiosis-specific nuclear structural 1 (MNS1) and intraflagellar transport 20 (IFT20), was verified by western blot. Functional clustering analysis indicated that these differentially expressed proteins were specifically enriched for terms such as spermatid development and flagellar assembly. Furthermore, we showed that SPEF2 interacts with radial spoke head component 9 (RSPH9) and IFT20 in vitro, which are well-studied components of radial spokes or intra-flagellar transport and are essential for flagellar assembly. These results provide a rich resource for further investigation into the molecular mechanism underlying the role that SPEF2 plays in sperm tail development and could provide a theoretical basis for gene therapy in SPEF2 mutant patients in the future.
Keywords: SPEF2; flagellar assembly; male infertility; protein interaction; proteomics.
Conflict of interest statement
None
Figures


Similar articles
-
SPEF2 functions in microtubule-mediated transport in elongating spermatids to ensure proper male germ cell differentiation.Development. 2017 Jul 15;144(14):2683-2693. doi: 10.1242/dev.152108. Epub 2017 Jun 15. Development. 2017. PMID: 28619825
-
Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein.Biol Reprod. 2010 Mar;82(3):580-90. doi: 10.1095/biolreprod.108.074971. Epub 2009 Nov 4. Biol Reprod. 2010. PMID: 19889948
-
Pathogenic gene variants in CCDC39, CCDC40, RSPH1, RSPH9, HYDIN, and SPEF2 cause defects of sperm flagella composition and male infertility.Front Genet. 2023 Feb 17;14:1117821. doi: 10.3389/fgene.2023.1117821. eCollection 2023. Front Genet. 2023. PMID: 36873931 Free PMC article.
-
Formation and function of the manchette and flagellum during spermatogenesis.Reproduction. 2016 Apr;151(4):R43-54. doi: 10.1530/REP-15-0310. Epub 2016 Jan 20. Reproduction. 2016. PMID: 26792866 Review.
-
Haploid male germ cells-the Grand Central Station of protein transport.Hum Reprod Update. 2020 Jun 18;26(4):474-500. doi: 10.1093/humupd/dmaa004. Hum Reprod Update. 2020. PMID: 32318721 Review.
Cited by
-
Sperm Phosphoproteome: Unraveling Male Infertility.Biology (Basel). 2022 Apr 25;11(5):659. doi: 10.3390/biology11050659. Biology (Basel). 2022. PMID: 35625387 Free PMC article. Review.
-
Genetic advancements and future directions in ruminant livestock breeding: from reference genomes to multiomics innovations.Sci China Life Sci. 2025 Apr;68(4):934-960. doi: 10.1007/s11427-024-2744-4. Epub 2024 Nov 26. Sci China Life Sci. 2025. PMID: 39609363 Review.
-
Novel SPEF2 Variant in a Japanese Patient with Primary Ciliary Dyskinesia: A Case Report and Literature Review.J Clin Med. 2022 Dec 31;12(1):317. doi: 10.3390/jcm12010317. J Clin Med. 2022. PMID: 36615117 Free PMC article.
-
Epididymal segment-specific miRNA and mRNA regulatory network at the single cell level.Cell Cycle. 2023 Oct;22(19):2194-2209. doi: 10.1080/15384101.2023.2280170. Epub 2023 Dec 5. Cell Cycle. 2023. PMID: 37982230 Free PMC article.
-
Population Structure and Selection Signatures Underlying Domestication Inferred from Genome-Wide Copy Number Variations in Chinese Indigenous Pigs.Genes (Basel). 2022 Nov 3;13(11):2026. doi: 10.3390/genes13112026. Genes (Basel). 2022. PMID: 36360263 Free PMC article.
References
-
- Tüttelmann F, Werny F, Cooper TG, Kliesch S, Simoni M, et al. Clinical experience with azoospermia: aetiology and chances for spermatozoa detection upon biopsy. Int J Androl. 2011;34:291–8. - PubMed
-
- Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15:369–84. - PubMed
-
- Adams JA, Galloway TS, Mondal D, Esteves SC, Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int. 2014;70:106–12. - PubMed
-
- Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–89. - PubMed
-
- Ortega C, Verheyen G, Raick D, Camus M, Devroey P, et al. Absolute asthenozoospermia and ICSI: what are the options? Hum Reprod Update. 2011;17:684–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

