Prospective Study of the Performance of Parent-Collected Nasal and Saliva Swab Samples, Compared with Nurse-Collected Swab Samples, for the Molecular Detection of Respiratory Microorganisms
- PMID: 34756077
- PMCID: PMC8579848
- DOI: 10.1128/Spectrum.00164-21
Prospective Study of the Performance of Parent-Collected Nasal and Saliva Swab Samples, Compared with Nurse-Collected Swab Samples, for the Molecular Detection of Respiratory Microorganisms
Abstract
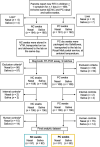
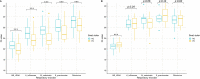
Respiratory tract infections (RTIs) are ubiquitous among children in the community. A prospective observational study was performed to evaluate the diagnostic performance and quality of at-home parent-collected (PC) nasal and saliva swab samples, compared to nurse-collected (NC) swab samples, from children with RTI symptoms. Children with RTI symptoms were swabbed at home on the same day by a parent and a nurse. We compared the performance of PC swab samples as the test with NC swab samples as the reference for the detection of respiratory pathogen gene targets by reverse transcriptase PCR, with quality assessment using a human gene. PC and NC paired nasal and saliva swab samples were collected from 91 and 92 children, respectively. Performance and interrater agreement (Cohen's κ) of PC versus NC nasal swab samples for viruses combined showed sensitivity of 91.6% (95% confidence interval [CI], 85.47 to 95.73%) and κ of 0.84 (95% CI, 0.79 to 0.88), respectively; the respective values for bacteria combined were 91.4% (95% CI, 86.85 to 94.87%) and κ of 0.85 (95% CI, 0.80 to 0.89). In saliva samples, viral and bacterial sensitivities were lower at 69.0% (95% CI, 57.47 to 79.76%) and 78.1% (95% CI, 71.60 to 83.76%), as were κ values at 0.64 (95% CI, 0.53 to 0.72) and 0.70 (95% CI, 0.65 to 0.76), respectively. Quality assessment for human biological material (18S rRNA) indicated perfect interrater agreement. At-home PC nasal swab samples performed comparably to NC swab samples, whereas PC saliva swab samples lacked sensitivity for the detection of respiratory microbes. IMPORTANCE RTIs are ubiquitous among children. Diagnosis involves a swab sample being taken by a health professional, which places a considerable burden on community health care systems, given the number of cases involved. The coronavirus disease 2019 (COVID-19) pandemic has seen an increase in the at-home self-collection of upper respiratory tract swab samples without the involvement of health professionals. It is advised that parents conduct or supervise swabbing of children. Surprisingly, few studies have addressed the quality of PC swab samples for subsequent identification of respiratory pathogens. We compared NC and PC nasal and saliva swab samples taken from the same child with RTI symptoms, for detection of respiratory pathogens. The PC nasal swab samples performed comparably to NC samples, whereas saliva swab samples lacked sensitivity for the detection of respiratory microbes. Collection of swab samples by parents would greatly reduce the burden on community nurses without reducing the effectiveness of diagnoses.
Keywords: clinical methods; community-based; diagnostics; microbiology; molecular techniques; parent collection; pediatric; public health; respiratory tract infection; self-collection.
Figures
Similar articles
-
The Respiratory Specimen Collection Trial (ReSpeCT): A Randomized Controlled Trial to Compare Quality and Timeliness of Respiratory Sample Collection in the Home by Parents and Healthcare Workers From Children Aged <2 Years.J Pediatric Infect Dis Soc. 2020 Apr 30;9(2):134-141. doi: 10.1093/jpids/piy136. J Pediatric Infect Dis Soc. 2020. PMID: 30657971 Free PMC article. Clinical Trial.
-
Side-by-side comparison of parent vs. technician-collected respiratory swabs in low-income, multilingual, urban communities in the United States.BMC Public Health. 2022 Jan 15;22(1):103. doi: 10.1186/s12889-022-12523-3. BMC Public Health. 2022. PMID: 35031041 Free PMC article.
-
Equivalence of self- and staff-collected nasal swabs for the detection of viral respiratory pathogens.PLoS One. 2012;7(11):e48508. doi: 10.1371/journal.pone.0048508. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155387 Free PMC article.
-
Accuracy and Acceptance of a Self-Collection Model for Respiratory Tract Infection Diagnostics: A Concise Clinical Literature Review.J Emerg Nurs. 2021 Sep;47(5):798-806. doi: 10.1016/j.jen.2021.04.006. Epub 2021 Jun 29. J Emerg Nurs. 2021. PMID: 34530972 Free PMC article. Review.
-
Use and evaluation of molecular diagnostics for pneumonia etiology studies.Clin Infect Dis. 2012 Apr;54 Suppl 2(Suppl 2):S153-8. doi: 10.1093/cid/cir1060. Clin Infect Dis. 2012. PMID: 22403230 Free PMC article. Review.
Cited by
-
A descriptive study on feasibility of nasopharyngeal and oropharyngeal swab collection from pediatric research participants in Cebu, Philippines.Front Public Health. 2025 Jun 10;13:1566688. doi: 10.3389/fpubh.2025.1566688. eCollection 2025. Front Public Health. 2025. PMID: 40556921 Free PMC article.
-
Human bocavirus-1 infections in Australian children aged < 2 years: a birth cohort study.Eur J Clin Microbiol Infect Dis. 2023 Jan;42(1):99-108. doi: 10.1007/s10096-022-04529-x. Epub 2022 Nov 25. Eur J Clin Microbiol Infect Dis. 2023. PMID: 36434280 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
Current State of Genomics in Nursing: A Scoping Review of Healthcare Provider Oriented (Clinical and Educational) Outcomes (2012-2022).Genes (Basel). 2023 Oct 27;14(11):2013. doi: 10.3390/genes14112013. Genes (Basel). 2023. PMID: 38002957 Free PMC article.
-
Acceptability, Feasibility, and Uptake of COVID-19 Antigen Rapid Diagnostic Self-Testing at the Community Level in Tanzania.Am J Trop Med Hyg. 2024 Nov 26;112(4_Suppl):26-36. doi: 10.4269/ajtmh.23-0732. Print 2025 Apr 1. Am J Trop Med Hyg. 2024. PMID: 39591640 Free PMC article.
References
-
- McCulloch DJ, Kim AE, Wilcox NC, Logue JK, Greninger AL, Englund JA, Chu HY. 2020. Comparison of unsupervised home self-collected midnasal swabs with clinician-collected nasopharyngeal swabs for detection of SARS-CoV-2 infection. JAMA Netw Open 3:e2016382. doi:10.1001/jamanetworkopen.2020.16382. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

