Type IV Pili of Streptococcus sanguinis Contribute to Pathogenesis in Experimental Infective Endocarditis
- PMID: 34756087
- PMCID: PMC8579931
- DOI: 10.1128/Spectrum.01752-21
Type IV Pili of Streptococcus sanguinis Contribute to Pathogenesis in Experimental Infective Endocarditis
Abstract
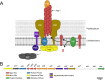
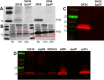
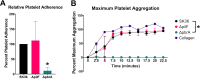
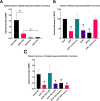
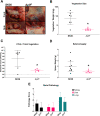
Streptococcus sanguinis is a common cause of infective endocarditis (IE). Efforts by research groups are aimed at identifying and characterizing virulence factors that contribute to the ability of this organism to cause IE. This Gram-positive pathogen causes heart infection by gaining access to the bloodstream, adhering to host extracellular matrix protein and/or platelets, colonizing the aortic endothelium, and incorporating itself into the aortic vegetation. While many virulence factors have been reported to contribute to the ability of S. sanguinis to cause IE, it is noteworthy that type IV pili (T4P) have not been described to be a virulence factor in this organism, although S. sanguinis strains typically encode these pili. Type IV pili are molecular machines that are capable of mediating diverse virulence functions and surface motility. T4P have been shown to mediate twitching motility in some strains of S. sanguinis, although in most strains it has been difficult to detect twitching motility. While we found that T4P are dispensable for direct in vitro platelet binding and aggregation phenotypes, we show that they are critical to the development of platelet-dependent biofilms representative of the cardiac vegetation. We also observed that T4P are required for in vitro invasion of S. sanguinis into human aortic endothelial cells, which indicates that S. sanguinis may use T4P to take advantage of an intracellular niche during infection. Importantly, we show that T4P of S. sanguinis are critical to disease progression (vegetation development) in a native valve IE rabbit model. The results presented here expand our understanding of IE caused by S. sanguinis and identify T4P as an important virulence factor for this pathogen. IMPORTANCE This work provides evidence that type IV pili produced by Streptococcus sanguinis SK36 are critical to the ability of these bacteria to attach to and colonize the aortic heart valve (endocarditis). We found that an S. sanguinis type IV pili mutant strain was defective in causing platelet-dependent aggregation in a 24-h infection assay but not in a 1-h platelet aggregation assay, suggesting that the type IV pili act at later stages of vegetation development. In a rabbit model of disease, a T4P mutant strain does not develop mature vegetations that form on the heart, indicating that this virulence factor is critical to disease and could be a target for IE therapy.
Keywords: Streptococcus sanguinis; bacterial pathogenesis; infective endocarditis; motility; platelet adherence; platelet aggregation; rabbit model; type IV pili.
Figures


Similar articles
-
Ecto-5'-nucleotidase: a candidate virulence factor in Streptococcus sanguinis experimental endocarditis.PLoS One. 2012;7(6):e38059. doi: 10.1371/journal.pone.0038059. Epub 2012 Jun 7. PLoS One. 2012. PMID: 22685551 Free PMC article.
-
Pili of oral Streptococcus sanguinis bind to fibronectin and contribute to cell adhesion.Biochem Biophys Res Commun. 2010 Jan 8;391(2):1192-6. doi: 10.1016/j.bbrc.2009.12.029. Epub 2009 Dec 14. Biochem Biophys Res Commun. 2010. PMID: 20004645
-
Molecular and Functional Analysis of the Type IV Pilus Gene Cluster in Streptococcus sanguinis SK36.Appl Environ Microbiol. 2019 Mar 6;85(6):e02788-18. doi: 10.1128/AEM.02788-18. Print 2019 Mar 15. Appl Environ Microbiol. 2019. PMID: 30635384 Free PMC article.
-
Platelet-streptococcal interactions in endocarditis.Crit Rev Oral Biol Med. 1996;7(3):222-36. doi: 10.1177/10454411960070030201. Crit Rev Oral Biol Med. 1996. PMID: 8909879 Review.
-
Biogenesis of Pseudomonas aeruginosa type IV pili and regulation of their function.Environ Microbiol. 2015 Nov;17(11):4148-63. doi: 10.1111/1462-2920.12849. Epub 2015 Jun 25. Environ Microbiol. 2015. PMID: 25808785 Review.
Cited by
-
Feature architecture aware phylogenetic profiling indicates a functional diversification of type IVa pili in the nosocomial pathogen Acinetobacter baumannii.PLoS Genet. 2023 Jul 27;19(7):e1010646. doi: 10.1371/journal.pgen.1010646. eCollection 2023 Jul. PLoS Genet. 2023. PMID: 37498819 Free PMC article.
-
Bacterial biofilms in the human body: prevalence and impacts on health and disease.Front Cell Infect Microbiol. 2023 Aug 30;13:1237164. doi: 10.3389/fcimb.2023.1237164. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37712058 Free PMC article. Review.
-
Phytochemical Analysis and Anti-Biofilm Potential That Cause Dental Caries from Black Cumin Seeds (Nigella sativa Linn.).Drug Des Devel Ther. 2024 May 29;18:1917-1932. doi: 10.2147/DDDT.S454217. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38828022 Free PMC article. Review.
-
Functional Analysis of the Major Pilin Proteins of Type IV Pili in Streptococcus sanguinis CGMH010.Int J Mol Sci. 2024 May 15;25(10):5402. doi: 10.3390/ijms25105402. Int J Mol Sci. 2024. PMID: 38791440 Free PMC article.
-
Bacterial interactions with platelets: defining key themes.Front Immunol. 2025 Jul 3;16:1610289. doi: 10.3389/fimmu.2025.1610289. eCollection 2025. Front Immunol. 2025. PMID: 40677721 Free PMC article. Review.
References
-
- Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VGJ, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falcó V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi:10.1001/archinternmed.2008.603. - DOI - PMC - PubMed
-
- Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA, American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi:10.1161/CIR.0000000000000296. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

