Differences in IgG Antibody Responses following BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines
- PMID: 34756093
- PMCID: PMC8579939
- DOI: 10.1128/Spectrum.01162-21
Differences in IgG Antibody Responses following BNT162b2 and mRNA-1273 SARS-CoV-2 Vaccines
Abstract
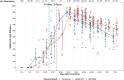
Studies examining antibody responses by vaccine brand are lacking and may be informative for optimizing vaccine selection, dosage, and regimens. The purpose of this study is to assess IgG antibody responses following immunization with BNT162b2 (30 μg mRNA) and mRNA-1273 (100 μg mRNA) vaccines. A cohort of clinicians at a nonprofit organization is being assessed clinically and serologically following immunization with BNT162b2 or mRNA-1273. IgG responses were measured at the Remington Laboratory by an IgG assay against the SARS-CoV-2 spike protein-receptor binding domain. Mixed-effect linear (MEL) regression modeling was used to examine whether the SARS-CoV-2 IgG level differed by vaccine brand, dosage, or number of days since vaccination. Among 532 SARS-CoV-2 seronegative participants, 530 (99.6%) seroconverted with either vaccine. After adjustments for age and gender, MEL regression modeling revealed that the average IgG antibody level increased after the second dose compared to the first dose (P < 0.001). Overall, titers peaked at week 6 for both vaccines. Titers were significantly higher for the mRNA-1273 vaccine on days 14 to 20 (P < 0.05), 42 to 48 (P < 0.01), 70 to 76 (P < 0.05), and 77 to 83 (P < 0.05) and higher for the BNT162b2 vaccine on days 28 to 34 (P < 0.001). In two participants taking immunosuppressive drugs, the SARS-CoV-2 IgG antibody response remained negative. mRNA-1273 elicited higher IgG antibody responses than BNT162b2, possibly due to the higher S-protein delivery. Prospective clinical and serological follow-up of defined cohorts such as this may prove useful in determining antibody protection and whether differences in antibody kinetics between the vaccines have manufacturing relevance and clinical significance. IMPORTANCE SARS-CoV-2 vaccines using the mRNA platform have become one of the most powerful tools to overcome the COVID-19 pandemic. mRNA vaccines enable human cells to produce and present the virus spike protein to their immune system, leading to protection from severe illness. Two mRNA vaccines have been widely implemented, mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech). We found that, following the second dose, spike protein antibodies were higher with mRNA-1273 than with BNT162b2. This is biologically plausible, since mRNA-1273 delivers a larger amount of mRNA (100 μg mRNA) than BNT162b2 (30 μg mRNA), which is translated into spike protein. This difference may need to be urgently translated into changes in the manufacturing process and dose regimens of these vaccines.
Keywords: BNT162b2; COVID-19; IgG; SARS-CoV-2; antibodies; mRNA-1273; vaccine; vaccines.
Figures
References
-
- Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, Talbot HK, Romero JR, Bell BP, Oliver SE. 2021. The Advisory Committee on Immunization Practices' updated interim recommendation for allocation of COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 69:1657–1660. doi: 10.15585/mmwr.mm695152e2. - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT, C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS. 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. doi: 10.1056/NEJMoa2035389. - DOI - PMC - PubMed
-
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ, Aboagye J, Adams K, Ali A, Allen E, Allison JL, Anslow R, Arbe-Barnes EH, Babbage G, Baillie K, Baker M, Baker N, Baker P, Baleanu I, Ballaminut J, Barnes E, Barrett J, Bates L, Batten A, et al. 2020. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396:467–478. doi: 10.1016/S0140-6736(20)31604-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous