Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study
- PMID: 34756184
- PMCID: PMC8555967
- DOI: 10.1016/S0140-6736(21)02249-2
Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study
Abstract
Background: Many countries are experiencing a resurgence of COVID-19, driven predominantly by the delta (B.1.617.2) variant of SARS-CoV-2. In response, these countries are considering the administration of a third dose of mRNA COVID-19 vaccine as a booster dose to address potential waning immunity over time and reduced effectiveness against the delta variant. We aimed to use the data repositories of Israel's largest health-care organisation to evaluate the effectiveness of a third dose of the BNT162b2 mRNA vaccine for preventing severe COVID-19 outcomes.
Methods: Using data from Clalit Health Services, which provides mandatory health-care coverage for over half of the Israeli population, individuals receiving a third vaccine dose between July 30, 2020, and Sept 23, 2021, were matched (1:1) to demographically and clinically similar controls who did not receive a third dose. Eligible participants had received the second vaccine dose at least 5 months before the recruitment date, had no previous documented SARS-CoV-2 infection, and had no contact with the health-care system in the 3 days before recruitment. Individuals who are health-care workers, live in long-term care facilities, or are medically confined to their homes were excluded. Primary outcomes were COVID-19-related admission to hospital, severe disease, and COVID-19-related death. The third dose effectiveness for each outcome was estimated as 1 - risk ratio using the Kaplan-Meier estimator.
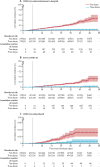
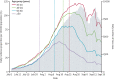
Findings: 1 158 269 individuals were eligible to be included in the third dose group. Following matching, the third dose and control groups each included 728 321 individuals. Participants had a median age of 52 years (IQR 37-68) and 51% were female. The median follow-up time was 13 days (IQR 6-21) in both groups. Vaccine effectiveness evaluated at least 7 days after receipt of the third dose, compared with receiving only two doses at least 5 months ago, was estimated to be 93% (231 events for two doses vs 29 events for three doses; 95% CI 88-97) for admission to hospital, 92% (157 vs 17 events; 82-97) for severe disease, and 81% (44 vs seven events; 59-97) for COVID-19-related death.
Interpretation: Our findings suggest that a third dose of the BNT162b2 mRNA vaccine is effective in protecting individuals against severe COVID-19-related outcomes, compared with receiving only two doses at least 5 months ago.
Funding: The Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests NB, ND, and RDB report institutional grants to Clalit Research Institute from Pfizer outside the submitted work and unrelated to COVID-19, with no direct or indirect personal benefits. MAH reports grants from the US National Institutes of Health (NIH) and US Department of Veterans Affairs, and personal fees from Cytel and ProPublica. ML reports grants from Pfizer, NIH, the UK National Institute for Health Research, the US Centers for Disease Control and Prevention, Open Philanthropy Project, the Wellcome Trust, and Pfizer; personal fees from Merck, Bristol Meyers Squibb, Sanofi Pasteur, and Janssen; and unpaid advice given on Covid vaccines or vaccine studies to One Day Sooner, Pfizer, AstraZeneca, Janssen, and COVAXX (United Biosciences), outside the submitted work. BYR reports grants from NIH outside the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Boosters appear effective, but are they always needed?Lancet. 2021 Dec 4;398(10316):2055-2057. doi: 10.1016/S0140-6736(21)02388-6. Epub 2021 Oct 29. Lancet. 2021. PMID: 34756185 Free PMC article. No abstract available.
-
Protection contre le Covid-19 après rappel (booster) par le vaccin Pfizer/BioNTech (BNT162b2).Rev Med Suisse. 2021 Dec 8;17(762):2154-2155. Rev Med Suisse. 2021. PMID: 34878747 French. No abstract available.
-
COVID-19 booster doses in pregnancy and global vaccine equity.Lancet. 2022 Mar 5;399(10328):907-908. doi: 10.1016/S0140-6736(22)00166-0. Epub 2022 Feb 18. Lancet. 2022. PMID: 35189078 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

