Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia
- PMID: 34756770
- PMCID: PMC8556135
- DOI: 10.1016/j.vaccine.2021.10.030
Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia
Abstract
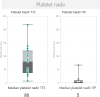
Emerging evidence suggest a possible association between immune thrombocytopenia (ITP) and some formulations of COVID-19 vaccine. We conducted a retrospective case series of ITP following vaccination with Vaxzevria ChadOx1-S (AstraZeneca) and mRNA Comirnaty BNT162b2 COVID-19 (Pfizer-BioNTech) vaccines and compare the incidence to expected background rates for Victoria during the first six months of the Australian COVID-19 vaccination roll-out in 2021. Cases were identified by reports to the Victorian state vaccine safety service, SAEFVIC, of individuals aged 18 years or older presenting with thrombocytopenia following COVID-19 vaccination without evidence of thrombosis. Twenty-one confirmed or probable cases of ITP were identified following receipt of AstraZeneca (n = 17) or Pfizer-BioNTech (n = 4) vaccines. This translates to an observed incidence of 8 per million doses for AstraZeneca vaccine, twice the expected background rate of 4.1 per million. The observed rate for Pfizer-BioNTech was consistent with the expected background rate. The median time to onset for the cases post AstraZeneca vaccination was 10 days (range 1-78) and median platelet nadir 5 × 109/L (range 0-67 × 109/L). Hospital presentations or admissions for management of symptoms such as bleeding occurred in 18 (86%) of the cases. The majority of cases (n = 11) required intervention with at least 2 therapy modalities. In conclusion, we observed a substantially higher than expected rate of ITP following AstraZeneca vaccination. ITP is the second haematological adverse event, distinct from that of thrombosis with thrombocytopenia syndrome (TTS), observed following AstraZeneca vaccination.
Keywords: COVID-19; Immune thrombocytopenia; Vaccination; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Operation COVID Shield. National COVID Vaccine Campaign Plan, https://www.health.gov.au/sites/default/files/documents/2021/08/op-covid... 2021 [accessed 13 August 2021].
-
- Therapeutic Goods Administration (TGA). Australian Product Information: COVID-19 Vaccine AstraZeneca (ChAdOx1-S) solution for injection, https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&a... 2021 [accessed 13 August 2021].
-
- Brighton Collaboration. Priority list of Adverse Events of Special Interest: COVID-19, https://brightoncollaboration.us/priority-list-aesi-covid/; 2020 [accessed 13 August 2021].
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical