Pleiotropic actions of IP6K1 mediate hepatic metabolic dysfunction to promote nonalcoholic fatty liver disease and steatohepatitis
- PMID: 34757046
- PMCID: PMC8609165
- DOI: 10.1016/j.molmet.2021.101364
Pleiotropic actions of IP6K1 mediate hepatic metabolic dysfunction to promote nonalcoholic fatty liver disease and steatohepatitis
Abstract
Objective: Obesity and insulin resistance greatly increase the risk of nonalcoholic fatty liver disease and steatohepatitis (NAFLD/NASH). We have previously discovered that whole-body and adipocyte-specific Ip6k1deletion protects mice from high-fat-diet-induced obesity and insulin resistance due to improved adipocyte thermogenesis and insulin signaling. Here, we aimed to determine the impact of hepatocyte-specific and whole-body Ip6k1 deletion (HKO and Ip6k1-KO or KO) on liver metabolism and NAFLD/NASH.
Methods: Body weight and composition; energy expenditure; glycemic profiles; and serum and liver metabolic, inflammatory, fibrotic and toxicity parameters were assessed in mice fed Western and high-fructose diet (HFrD) (WD: 40% kcal fat, 1.25% cholesterol, no added choline and HFrD: 60% kcal fructose). Mitochondrial oxidative capacity was evaluated in isolated hepatocytes. RNA-Seq was performed in liver samples. Livers from human NASH patients were analyzed by immunoblotting and mass spectrometry.
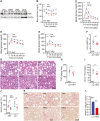
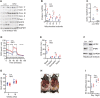
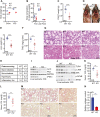
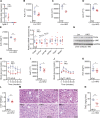
Results: HKO mice displayed increased hepatocyte mitochondrial oxidative capacity and improved insulin sensitivity but were not resistant to body weight gain. Improved hepatocyte metabolism partially protected HKO mice from NAFLD/NASH. In contrast, enhanced whole-body metabolism and reduced body fat accumulation significantly protected whole-body Ip6k1-KO mice from NAFLD/NASH. Mitochondrial oxidative pathways were upregulated, whereas gluconeogenic and fibrogenic pathways were downregulated in Ip6k1-KO livers. Furthermore, IP6K1 was upregulated in human NASH livers and interacted with the enzyme O-GlcNAcase that reduces protein O-GlcNAcylation. Protein O-GlcNAcylation was found to be reduced in Ip6k1-KO and HKO mouse livers.
Conclusion: Pleiotropic actions of IP6K1 in the liver and other metabolic tissues mediate hepatic metabolic dysfunction and NAFLD/NASH, and thus IP6K1 deletion may be a potential treatment target for this disease.
Keywords: Hyperglycemia; IP6K1; Liver; Metabolism; NAFLD/NASH; O-GlcNAcylation.
Copyright © 2021 The Author(s). Published by Elsevier GmbH.. All rights reserved.
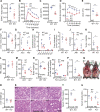
Figures




References
-
- Saiardi A. Cell signalling by inositol pyrophosphates. Subcellular Biochemistry. 2012;59:413–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

