"ADPKD-omics": determinants of cyclic AMP levels in renal epithelial cells
- PMID: 34757121
- PMCID: PMC10671900
- DOI: 10.1016/j.kint.2021.10.014
"ADPKD-omics": determinants of cyclic AMP levels in renal epithelial cells
Abstract
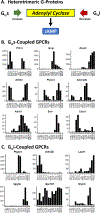
The regulation of cyclic adenosine monophosphate (cAMP) levels in kidney epithelial cells is important in at least 2 groups of disorders, namely water balance disorders and autosomal dominant polycystic kidney disease. Focusing on the latter, we review genes that code for proteins that are determinants of cAMP levels in cells. We identify which of these determinants are expressed in the 14 kidney tubule segments using recently published RNA-sequencing and protein mass spectrometry data ("autosomal dominant polycystic kidney disease-omics"). This includes G protein-coupled receptors, adenylyl cyclases, cyclic nucleotide phosphodiesterases, cAMP transporters, cAMP-binding proteins, regulator of G protein-signaling proteins, G protein-coupled receptor kinases, arrestins, calcium transporters, and calcium-binding proteins. In addition, compartmentalized cAMP signaling in the primary cilium is discussed, and a specialized database of the proteome of the primary cilium of cultured "IMCD3" cells is provided as an online resource (https://esbl.nhlbi.nih.gov/Databases/CiliumProteome/). Overall, this article provides a general resource in the form of a curated list of proteins likely to play roles in determination of cAMP levels in kidney epithelial cells and, therefore, likely to be determinants of progression of autosomal dominant polycystic kidney disease.
Keywords: RNA sequencing; protein mass spectrometry; vaptans; vasopressin.
Published by Elsevier Inc.
Figures








References
-
- Padovano V, Podrini C, Boletta A, et al. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat Rev Nephrol 2018; 14: 678–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

