Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs
- PMID: 34757287
- PMCID: PMC8502116
- DOI: 10.1016/j.dmpk.2021.100424
Difference in the lipid nanoparticle technology employed in three approved siRNA (Patisiran) and mRNA (COVID-19 vaccine) drugs
Abstract
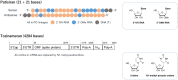
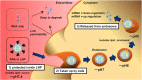
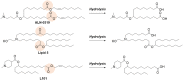
Nucleic acid therapeutics are developing into precise medicines that can manipulate specific genes. However, the development of safe and effective delivery system for the target cells has remained a challenge. Lipid nanoparticles (LNPs) have provided a revolutionary delivery system that can ensure multiple clinical translation of RNA-based candidates. In 2018, Patisiran (Onpattro) was first approved as an LNP-based siRNA drug. In 2020, during the coronavirus disease 2019 (COVID-19) outbreak, LNPs have enabled the development of two SARS-CoV-2 mRNA vaccines, Tozinameran (Comirnaty or Pfizer-BioNTech COVID-19 vaccine) and Elasomeran (Spikevax or COVID-19 vaccine Moderna) for conditional approval. Here, we reviewed the state-of-the-art LNP technology employed in three approved drugs (one siRNA-based and two mRNA-based drugs) and discussed the differences in their mode of action, formulation design, and biodistribution.
Keywords: COVID-19; COVID-19 vaccine moderna; Elasomeran; Ionizable lipid; LNP; Lipid nanoparticles; Patisiran; SARS-CoV-2; Tozinameran; mRNA vaccine.
Copyright © 2021 The Japanese Society for the Study of Xenobiotics. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no conflicts of interest associated with this manuscript.
Figures

Similar articles
-
Delivery Strategies for mRNA Vaccines.Pharmaceut Med. 2022 Feb;36(1):11-20. doi: 10.1007/s40290-021-00417-5. Epub 2022 Jan 30. Pharmaceut Med. 2022. PMID: 35094366 Free PMC article. Review.
-
Langerhans cells and cDC1s play redundant roles in mRNA-LNP induced protective anti-influenza and anti-SARS-CoV-2 immune responses.PLoS Pathog. 2022 Jan 24;18(1):e1010255. doi: 10.1371/journal.ppat.1010255. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35073387 Free PMC article.
-
Production and Evaluation of Nucleoside-Modified mRNA Vaccines for Infectious Diseases.Methods Mol Biol. 2024;2786:167-181. doi: 10.1007/978-1-0716-3770-8_7. Methods Mol Biol. 2024. PMID: 38814394
-
From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases.Acta Biomater. 2021 Sep 1;131:16-40. doi: 10.1016/j.actbio.2021.06.023. Epub 2021 Jun 18. Acta Biomater. 2021. PMID: 34153512 Free PMC article. Review.
-
A Basic Method for Formulating mRNA-Lipid Nanoparticle Vaccines in the Lab.Methods Mol Biol. 2024;2786:237-254. doi: 10.1007/978-1-0716-3770-8_11. Methods Mol Biol. 2024. PMID: 38814398
Cited by
-
Investigation of the ionic conditions in SiRNA-mediated delivery through its carriers in the cell membrane: a molecular dynamic simulation.Sci Rep. 2022 Oct 20;12(1):17520. doi: 10.1038/s41598-022-22509-1. Sci Rep. 2022. PMID: 36266467 Free PMC article.
-
miR-142: A Master Regulator in Hematological Malignancies and Therapeutic Opportunities.Cells. 2023 Dec 30;13(1):84. doi: 10.3390/cells13010084. Cells. 2023. PMID: 38201290 Free PMC article. Review.
-
Nucleic Acids as Biotools at the Interface between Chemistry and Nanomedicine in the COVID-19 Era.Int J Mol Sci. 2022 Apr 14;23(8):4359. doi: 10.3390/ijms23084359. Int J Mol Sci. 2022. PMID: 35457177 Free PMC article. Review.
-
Delivery of Lipid Nanoparticles with ROS Probes for Improved Visualization of Hepatocellular Carcinoma.Biomedicines. 2023 Jun 21;11(7):1783. doi: 10.3390/biomedicines11071783. Biomedicines. 2023. PMID: 37509423 Free PMC article.
-
Recent Advancement in mRNA Vaccine Development and Applications.Pharmaceutics. 2023 Jul 18;15(7):1972. doi: 10.3390/pharmaceutics15071972. Pharmaceutics. 2023. PMID: 37514158 Free PMC article. Review.
References
-
- Takakusa H., Iwazaki N., Nishikawa M., Yoshida T., Obika S., Inoue T. Drug metabolism & pharmacokinetics of oligonucleotide therapeutics : profiles and evaluation approaches. Pharmaceutical and Medical Device Regulatory Science. 2021;52(2):76–84.
-
- Sato Y., Hatakeyama H., Sakurai Y., Hyodo M., Akita H., Harashima H. A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J Contr Release. 2012;163(3):267–276. - PubMed
-
- Akita H., Noguchi Y., Hatakeyama H., Sato Y., Tange K., Nakai Y., et al. Molecular tuning of a vitamin E-scaffold pH-sensitive and reductive cleavable lipid-like material for accelerated in vivo hepatic siRNA delivery. ACS Biomater Sci Eng. 2015;1(9):834–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

