Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats
- PMID: 34757568
- PMCID: PMC8811093
- DOI: 10.1007/s11357-021-00483-2
Mesenchymal stromal cell-derived small extracellular vesicles promote neurological recovery and brain remodeling after distal middle cerebral artery occlusion in aged rats
Abstract
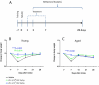
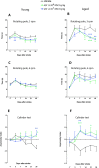
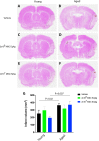
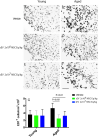
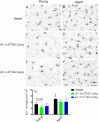
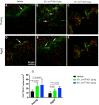
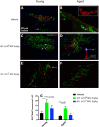
Small extracellular vesicles (sEVs) obtained from mesenchymal stromal cells (MSCs) promote neurological recovery after middle cerebral artery occlusion (MCAO) in young rodents. Ischemic stroke mainly affects aged humans. MSC-sEV effects on stroke recovery in aged rodents had not been assessed. In a head-to-head comparison, we exposed young (4-5 months) and aged (19-20 months) male Sprague-Dawley rats to permanent distal MCAO. At 24 h, 3 and 7 days post-stroke, vehicle or MSC-sEVs (2 × 106 or 2 × 107 MSC equivalents/kg) were intravenously administered. Neurological deficits, ischemic injury, brain inflammatory responses, post-ischemic angiogenesis, and endogenous neurogenesis were evaluated over 28 days. Post-MCAO, aged vehicle-treated rats exhibited more severe motor-coordination deficits evaluated by rotating pole and cylinder tests and larger brain infarcts than young vehicle-treated rats. Although infarct volume was not influenced by MSC-sEVs, sEVs at both doses effectively reduced motor-coordination deficits in young and aged rats. Brain macrophage infiltrates in periinfarct tissue, which were evaluated as marker of a recovery-aversive inflammatory environment, were significantly stronger in aged than young vehicle-treated rats. sEVs reduced brain macrophage infiltrates in aged, but not young rats. The tolerogenic shift in immune balance paved the way for structural brain tissue remodeling. Hence, sEVs at both doses increased periinfarct angiogenesis evaluated by CD31/BrdU immunohistochemistry in young and aged rats, and low-dose sEVs increased neurogenesis in the subventricular zone examined by DCX/BrdU immunohistochemistry. Our study provides robust evidence that MSC-sEVs promote functional neurological recovery and brain tissue remodeling in aged rats post-stroke. This study encourages further proof-of-concept studies in clinic-relevant stroke settings.
Keywords: Aging; Angiogenesis; Exosome; Ischemic stroke; Macrophage; Neurogenesis; Permanent focal cerebral ischemia; Stem/precursor cell.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

