Quality of Life in Men With Prostate Cancer Randomly Allocated to Receive Docetaxel or Abiraterone in the STAMPEDE Trial
- PMID: 34757812
- PMCID: PMC7612717
- DOI: 10.1200/JCO.21.00728
Quality of Life in Men With Prostate Cancer Randomly Allocated to Receive Docetaxel or Abiraterone in the STAMPEDE Trial
Abstract
Purpose: Docetaxel and abiraterone acetate plus prednisone or prednisolone (AAP) both improve survival when commenced alongside standard of care (SOC) androgen deprivation therapy in locally advanced or metastatic hormone-sensitive prostate cancer. Thus, patient-reported quality of life (QOL) data may guide treatment choices.
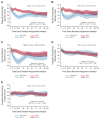
Methods: A group of patients within the STAMPEDE trial were contemporaneously enrolled with the possibility of being randomly allocated to receive either docetaxel + SOC or AAP + SOC. A mixed-model assessed QOL in those who had completed at least one QLQ-C30 + PR25 questionnaire. The primary outcome measure was difference in global-QOL (QLQ-C30 Q29&30) between patients allocated to docetaxel + SOC or AAP + SOC over the 2 years after random assignment, with a predefined criterion for clinically meaningful difference of > 4.0 points. Secondary outcome measures included longitudinal comparison of functional domains, pain, and fatigue, plus global-QOL at defined timepoints.
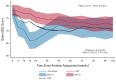
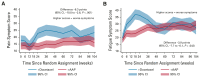
Results: Five hundred fifteen patients (173 docetaxel + SOC and 342 AAP + SOC) were included. Baseline characteristics, proportion of missing data, and mean baseline global-QOL scores (docetaxel + SOC 77.8 and AAP + SOC 78.0) were similar. Over the 2 years following random assignment, the mean modeled global-QOL score was +3.9 points (95% CI, +0.5 to +7.2; P = .022) higher in patients allocated to AAP + SOC. Global-QOL was higher for patients allocated to AAP + SOC over the first year (+5.7 points, 95% CI, +3.0 to +8.5; P < .001), particularly at 12 (+7.0 points, 95% CI, +3.0 to +11.0; P = .001) and 24 weeks (+8.3 points, 95% CI, +4.0 to +12.6; P < .001).
Conclusion: Patient-reported QOL was superior for patients allocated to receive AAP + SOC, compared with docetaxel + SOC over a 2-year period, narrowly missing the predefined value for clinical significance. Patients receiving AAP + SOC reported clinically meaningful higher global-QOL scores throughout the first year following random assignment.
Trial registration: ClinicalTrials.gov NCT00268476.
Conflict of interest statement
Figures
Comment in
-
Elevating the Patient Voice in Metastatic Hormone-Sensitive Prostate Cancer Clinical Trials.J Clin Oncol. 2022 Mar 10;40(8):807-810. doi: 10.1200/JCO.21.02504. Epub 2022 Jan 6. J Clin Oncol. 2022. PMID: 34990219 No abstract available.
References
-
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020;77:508–547. - PubMed
-
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 12459/CRUK_/Cancer Research UK/United Kingdom
- 28284/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00004/02/MRC_/Medical Research Council/United Kingdom
- 20942/CRUK_/Cancer Research UK/United Kingdom
- DH_/Department of Health/United Kingdom
- 3804/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_00004/01/MRC_/Medical Research Council/United Kingdom
- 12518/CRUK_/Cancer Research UK/United Kingdom
- A3804/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12023/25/MRC_/Medical Research Council/United Kingdom
- 19727/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

