Streamlined Whole-Genome Sequencing of Mumps Virus for High-Resolution Outbreak Analysis
- PMID: 34757832
- PMCID: PMC8769722
- DOI: 10.1128/JCM.00841-21
Streamlined Whole-Genome Sequencing of Mumps Virus for High-Resolution Outbreak Analysis
Abstract
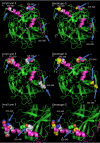
Since 2015, the United States has experienced a resurgence in the number of mumps cases and outbreaks in fully vaccinated populations. These outbreaks have occurred predominantly in close-quarter settings, such as camps, colleges, and detention centers. Phylogenetic analysis of 758 mumps-positive samples from outbreaks across the United States identified 743 (98%) as genotype G based on sequence analysis of the mumps small hydrophobic (SH) gene. Additionally, SH sequences in the genotype G samples showed almost no sequence diversity, with 675 (91%) of them having identical sequences or only one nucleotide difference. This uniformity of circulating genotype and strain created complications for epidemiologic investigations and necessitated the development of a system for rapidly generating mumps whole-genome sequences for more detailed analysis. In this study, we report a novel and streamlined assay for whole-genome sequencing (WGS) of mumps virus genotype G. The WGS procedure successfully generated 318 high-quality WGS sequences on nucleic acid from genotype G-positive respiratory samples collected during several mumps outbreaks in the United States between 2016 and 2019. Sequencing was performed by a rapid and highly sensitive custom Ion AmpliSeq mumps genotype G panel, with sample preparation performed on an Ion Chef and sequencing on an Ion S5. The WGS data generated by the AmpliSeq panel provided enhanced genomic resolution for epidemiological outbreak investigations. Translation and protein sequence analysis also identified several potentially important epitope changes in the circulating mumps genotype G strains compared to the Jeryl-Lynn strain (JL5) used in vaccines in the United States, which could explain the current level of vaccine escapes.
Keywords: AmpliSeq; genotyping; molecular epidemiology; mumps; vaccine; whole-genome sequencing.
Conflict of interest statement
P.B., H.C., D.M.L., and T.Y. declare no competing interests. K.S.G. receives research support from ThermoFisher for the evaluation of new assays for the detection and characterization of viruses. She also has a royalty-generating collaborative agreement with Zeptometrix.
Figures


References
-
- Jin L, Rima B, Brown D, Orvell C, Tecle T, Afzal M, Uchida K, Nakayama T, Song JW, Kang C, Rota PA, Xu W, Featherstone D. 2005. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch Virol 150:1903–1909. 10.1007/s00705-005-0563-4. - DOI - PubMed
-
- Centers for Disease Control and Prevention. 1978. Mumps surveillance-United States. MMWR Morb Mortal Wkly Rep 40:379–381.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

