The M2 Gene Is a Determinant of Reovirus-Induced Myocarditis
- PMID: 34757847
- PMCID: PMC8791294
- DOI: 10.1128/JVI.01879-21
The M2 Gene Is a Determinant of Reovirus-Induced Myocarditis
Abstract
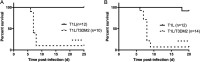
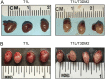
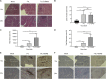
Although a broad range of viruses cause myocarditis, the mechanisms that underlie viral myocarditis are poorly understood. Here, we report that the M2 gene is a determinant of reovirus myocarditis. The M2 gene encodes outer capsid protein μ1, which mediates host membrane penetration during reovirus entry. We infected newborn C57BL/6 mice with reovirus strain type 1 Lang (T1L) or a reassortant reovirus in which the M2 gene from strain type 3 Dearing (T3D) was substituted into the T1L genetic background (T1L/T3DM2). T1L was nonlethal in wild-type mice, whereas more than 90% of mice succumbed to T1L/T3DM2 infection. T1L/T3DM2 produced higher viral loads than T1L at the site of inoculation. In secondary organs, T1L/T3DM2 was detected with more rapid kinetics and reached higher peak titers than T1L. We found that hearts from T1L/T3DM2-infected mice were grossly abnormal, with large lesions indicative of substantial inflammatory infiltrate. Lesions in T1L/T3DM2-infected mice contained necrotic cardiomyocytes with pyknotic debris, as well as extensive lymphocyte and histiocyte infiltration. In contrast, T1L induced the formation of small purulent lesions in a small subset of animals, consistent with T1L being mildly myocarditic. Finally, more activated caspase-3-positive cells were observed in hearts from animals infected with T1L/T3DM2 than T1L. Together, our findings indicate that substitution of the T3D M2 allele into an otherwise T1L genetic background is sufficient to change a nonlethal infection into a lethal infection. Our results further indicate that T3D M2 enhances T1L replication and dissemination in vivo, which potentiates the capacity of reovirus to cause myocarditis. IMPORTANCE Reovirus is a nonenveloped virus with a segmented double-stranded RNA genome that serves as a model for studying viral myocarditis. The mechanisms by which reovirus drives myocarditis development are not fully elucidated. We found that substituting the M2 gene from strain type 3 Dearing (T3D) into an otherwise type 1 Lang (T1L) genetic background (T1L/T3DM2) was sufficient to convert the nonlethal T1L strain into a lethal infection in neonatal C57BL/6 mice. T1L/T3DM2 disseminated more efficiently and reached higher maximum titers than T1L in all organs tested, including the heart. T1L is mildly myocarditic and induced small areas of cardiac inflammation in a subset of mice. In contrast, hearts from mice infected with T1L/T3DM2 contained extensive cardiac inflammatory infiltration and more activated caspase-3-positive cells, which is indicative of apoptosis. Together, our findings identify the reovirus M2 gene as a new determinant of reovirus-induced myocarditis.
Keywords: dissemination; inflammation; myocarditis; pathogenesis; reovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Duechting A, TschöPe C, Kaiser H, Lamkemeyer T, Tanaka N, Aberle S, Lang F, Torresi J, Kandolf R, Bock C-T. 2008. Human parvovirus B19 NS1 protein modulates inflammatory signaling by activation of STAT3/PIAS3 in human endothelial cells. J Virol 82:7942–7952. 10.1128/JVI.00891-08. - DOI - PMC - PubMed
-
- Perez-Bermejo JA, Kang S, Rockwood SJ, Simoneau CR, Joy DA, Silva AC, Ramadoss GN, Flanigan WR, Fozouni P, Li H, Chen PY, Nakamura K, Whitman JD, Hanson PJ, McManus BM, Ott M, Conklin BR, McDevitt TC. 2021. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci Transl Med 13:eabf7872. 10.1126/scitranslmed.abf7872. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

