A SARS-CoV-2 coronavirus nucleocapsid protein antigen-detecting lateral flow assay
- PMID: 34758052
- PMCID: PMC8580225
- DOI: 10.1371/journal.pone.0258819
A SARS-CoV-2 coronavirus nucleocapsid protein antigen-detecting lateral flow assay
Abstract
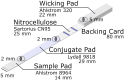
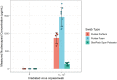
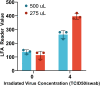
Inexpensive, simple, rapid diagnostics are necessary for efficient detection, treatment, and mitigation of COVID-19. Assays for SARS-CoV2 using reverse transcription polymerase chain reaction (RT-PCR) offer good sensitivity and excellent specificity, but are expensive, slowed by transport to centralized testing laboratories, and often unavailable. Antigen-based assays are inexpensive and can be rapidly mass-produced and deployed at point-of-care, with lateral flow assays (LFAs) being the most common format. While various manufacturers have produced commercially available SARS-Cov2 antigen LFAs, access to validated tests remains difficult or cost prohibitive in low-and middle-income countries. Herein, we present a visually read open-access LFA (OA-LFA) using commercially-available antibodies and materials for the detection of SARS-CoV-2. The LFA yielded a Limit of Detection (LOD) of 4 TCID50/swab of gamma irradiated SARS-CoV-2 virus, meeting the acceptable analytical sensitivity outlined by in World Health Organization target product profile. The open-source architecture presented in this manuscript provides a template for manufacturers around the globe to rapidly design a SARS-CoV2 antigen test.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Weekly epidemiological update on COVID-19–31 August 2021. 2021 [cited 2 Sep 2021]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

