Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): Results of a post-approval observational study
- PMID: 34758142
- PMCID: PMC9299719
- DOI: 10.1111/dme.14739
Non-adjunctive continuous glucose monitoring for control of hypoglycaemia (COACH): Results of a post-approval observational study
Abstract
Objective: Prior to the Continuous Monitoring and Control of Hypoglycaemia (COACH) study described herein, no study had been powered to evaluate the impact of non-adjunctive RT-CGM use on the rate of debilitating moderate or severe hypoglycaemic events.
Research design and methods: In this 12-month observational study, adults with insulin-requiring diabetes who were new to RT-CGM participated in a 6-month control phase where insulin dosing decisions were based on self monitoring of blood glucose values, followed by a 6-month phase where decisions were based on RT-CGM data (i.e. non-adjunctive RT-CGM use); recommendations for RT-CGM use were made according to sites' usual care. The primary outcome was change in debilitating moderate (requiring second-party assistance) and severe (resulting in seizures or loss of consciousness) hypoglycaemic event frequency. Secondary outcomes included changes in HbA1c and diabetic ketoacidosis (DKA) frequency.
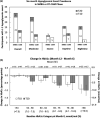
Results: A total of 519 participants with mean (SD) age 50.3 (16.1) years and baseline HbA1c 8.0% (1.4%) completed the study, of whom 32.8% had impaired hypoglycaemia awareness and 33.5% had type 2 diabetes (T2D). The mean (SE) per-patient frequency of hypoglycaemic events decreased by 63% from 0.08 (0.016) during the SMBG phase to 0.03 (0.010) during the RT-CGM phase (p = 0.005). HbA1c decreased during the RT-CGM phase both for participants with type 1 diabetes (T1D) and T2D and there was a trend towards larger reductions among individuals with higher baseline HbA1c.
Conclusions: Among adults with insulin-requiring diabetes, non-adjunctive use of RT-CGM data is safe, resulting in significantly fewer debilitating hypoglycaemic events than management using SMBG.
Keywords: COACH; continuous glucose monitoring; severe hypoglycaemia.
© 2021 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Conflict of interest statement
Drs. Beck and Price are employees and shareholders of Dexcom, Inc. Dr. Kelly has received consulting fees from Dexcom, Inc.
Figures
References
-
- Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non‐insulin‐dependent diabetes mellitus: a randomized prospective 6‐year study. Diabetes Res Clin Pract. 1995;28(2):103‐117. - PubMed
-
- The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. - PubMed
-
- DCCT Research Group . Hypoglycemia in the diabetes control and complications trial. The diabetes control and complications trial research group. Diabetes. 1997;46(2):271‐286. - PubMed
-
- Mathieu C. Minimising hypoglycaemia in the real world: the challenge of insulin. Diabetologia. 2021;64(5):978‐984. - PubMed
-
- Bode BW, Schwartz S, Stubbs HA, Block JE. Glycemic characteristics in continuously monitored patients with type 1 and type 2 diabetes: normative values. Diabetes Care. 2005;28(10):2361‐2366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

