Polyneuropathy monitoring in Parkinson's disease patients treated with levodopa/carbidopa intestinal gel
- PMID: 34758207
- PMCID: PMC8671764
- DOI: 10.1002/brb3.2408
Polyneuropathy monitoring in Parkinson's disease patients treated with levodopa/carbidopa intestinal gel
Abstract
Objectives: Levodopa-carbidopa-intestinal-gel (LCIG) infusion is an effective treatment for advanced PD with motor fluctuations. Polyneuropathy occurs as a complication in 10-15% of patients. We wanted to assess the frequency of polyneuropathy in Finnish advanced Parkinson's disease (PD) patients with continuous LCIG infusion, and the value of different clinical monitoring parameters during follow-up.
Materials and methods: Patient records of PD patients started on LCIG infusion at Helsinki University Hospital who received nerve conduction studies at baseline and 6 months after treatment initiation were reviewed for epidemiological information, mini mental state examination, baseline and 6 months' UPRDS-III, weight, body mass index, levodopa dose (LD), plasma homocysteine levels, folate, vitamin B6 and B12.
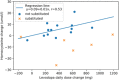
Results: Out of 19 patients (n = 6 on B-vitamin substitution), two (10.5%) developed new-onset polyneuropathy after initiation of LCIG therapy (n = 0 with vitamin substitution). Neuropathy was associated with significant weight loss (BMI reduction > 1.5), but not with other monitoring parameters. Homocysteine rose significantly in patients not substituted with B-vitamin complex, but not in patients with B-vitamin substitution. Homocysteine changes correlated with LD changes in the absence of vitamin B substitution. After oral B-vitamin substitution, both patients' polyneuropathy remained electrophysiologically and clinically stable.
Conclusions: Rates of polyneuropathy in Finnish PD patients with LCIG treatment are comparable to previous studies. Patients' weight should be included in regular follow up monitoring and can be used for patient self-monitoring. Vitamin B substitution appears to reduce coupling between levodopa dose and homocysteine and may be useful to prevent polyneuropathy related to LCIG.
Keywords: LCIG; Parkinson's disease; levodopa infusion; polyneuropathy.
© 2021 The Authors. Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
Amande Pauls has received lecture fees from Abbvie. Jussi Toppila has received lecture fees from UCB Pharma and is a consulting medical expert for SGS Fimko Ltd. Maija Koivu has received lecture fees from Abbvie. Johanna Eerola‐Rautio has received lecture fees from Abbvie, Nordicinfu Care AB and Allergan, and is a member of the advisory board of Nordicinfu Care AB. Marianne Udd has received lecture fees from Abbvie. Eero Pekkonen reports the following disclosures: Consulting fees by NordicInfu Care AB, Abbvie. Member of the advisory board: Abbvie. Lecturing fees: Abbott, Abbvie. Consulting neurologist for Finnish Patient Insurance Centre. Standing member of the MDS Non‐Motor Parkinson's Disease Study Group. Person Responsible of Trial in Finland: International DYSCOVER‐study (Dyskinesia Comparative Interventional Trial on Duodopa vs. oral medication) 2017–19, organized by Abbvie.
Figures

References
-
- Olanow, C. W. , Kieburtz, K. , Odin, P. , Espay, A. J. , Standaert, D. G. , Fernandez, H. H. , Vanagunas, A. , Othman, A. A. , Widnell, K. L. , Robieson, W. Z. , Pritchett, Y. , Chatamra, K. , Benesh, J. , Lenz, R. A. , & Antonini, A. (2014). Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: A randomised, controlled, double‐blind, double‐dummy study. The Lancet Neurology, 13, 141–149. 10.1016/S1474-4422(13)70293-X - DOI - PMC - PubMed
-
- Fernandez, H. H. , Standaert, D. G. , Hauser, R. A. , Lang, A. E. , Fung, V. S. C. , Klostermann, F. , Lew, M. F. , Odin, P. , Steiger, M. , Yakupov, E. Z. , Chouinard, S. , Suchowersky, O. , Dubow, J. , Hall, C. M. , Chatamra, K. , Robieson, W. Z. , Benesh, J. A. , & Espay, A. J. (2015). Levodopa‐carbidopa intestinal gel in advanced Parkinson's disease: Final 12‐month, open‐label results. Movement Disorders, 30, 500–509. 10.1002/mds.26123 - DOI - PMC - PubMed
-
- Antonini, A. , Poewe, W. , Chaudhuri, K. R. , Jech, R. , Pickut, B. , Pirtošek, Z. , Szasz, J. , Valldeoriola, F. , Winkler, C. , Bergmann, L. , Yegin, A. , Onuk, K. , Barch, D. , Odin, P. , Amalia, E. , Arnold, G. , Bajenaru, O. , Bergmans, B. , Bjornara, K. A. , …, Van Zandijcke, M. (2017). Levodopa‐carbidopa intestinal gel in advanced Parkinson's: Final results of the GLORIA registry. Parkinsonism & Related Disorders, 45, 13–20. 10.1016/j.parkreldis.2017.09.018 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

