Distinct in vivo dynamics of excitatory synapses onto cortical pyramidal neurons and parvalbumin-positive interneurons
- PMID: 34758304
- PMCID: PMC8631347
- DOI: 10.1016/j.celrep.2021.109972
Distinct in vivo dynamics of excitatory synapses onto cortical pyramidal neurons and parvalbumin-positive interneurons
Abstract
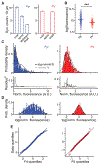
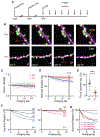
Cortical function relies on the balanced activation of excitatory and inhibitory neurons. However, little is known about the organization and dynamics of shaft excitatory synapses onto cortical inhibitory interneurons. Here, we use the excitatory postsynaptic marker PSD-95, fluorescently labeled at endogenous levels, as a proxy for excitatory synapses onto layer 2/3 pyramidal neurons and parvalbumin-positive (PV+) interneurons in the barrel cortex of adult mice. Longitudinal in vivo imaging under baseline conditions reveals that, although synaptic weights in both neuronal types are log-normally distributed, synapses onto PV+ neurons are less heterogeneous and more stable. Markov model analyses suggest that the synaptic weight distribution is set intrinsically by ongoing cell-type-specific dynamics, and substantial changes are due to accumulated gradual changes. Synaptic weight dynamics are multiplicative, i.e., changes scale with weights, although PV+ synapses also exhibit an additive component. These results reveal that cell-type-specific processes govern cortical synaptic strengths and dynamics.
Keywords: Markov model; PSD-95; additive and multiplicative synaptic dynamics; in vivo two-photon imaging; log normality; parvalbumin-positive inhibitory interneuron; shaft excitatory synapses; structural plasticity; synaptic weight.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.Z. is the inventor of the PSD-95-ENABLED mouse, which has been licensed to several companies. This potential conflict of interest has been reviewed and managed by OHSU.
Figures


References
-
- Bergmann I, Nitsch R, and Frotscher M (1991). Area-specific morphological and neurochemical maturation of non-pyramidal neurons in the rat hippocampus as revealed by parvalbumin immunocytochemistry. Anat. Embryol. (Berl) 184, 403–409. - PubMed
-
- Bhatt DH, Zhang S, and Gan WB (2009). Dendritic spine dynamics. Annu. Rev. Physiol 71, 261–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

