Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation
- PMID: 34759002
- PMCID: PMC8579424
- DOI: 10.1136/bmj-2021-066542
Exercise versus usual care after non-reconstructive breast cancer surgery (UK PROSPER): multicentre randomised controlled trial and economic evaluation
Abstract
Objective: To evaluate whether a structured exercise programme improved functional and health related quality of life outcomes compared with usual care for women at high risk of upper limb disability after breast cancer surgery.
Design: Multicentre, pragmatic, superiority, randomised controlled trial with economic evaluation.
Setting: 17 UK National Health Service cancer centres.
Participants: 392 women undergoing breast cancer surgery, at risk of postoperative upper limb morbidity, randomised (1:1) to usual care with structured exercise (n=196) or usual care alone (n=196).
Interventions: Usual care (information leaflets) only or usual care plus a physiotherapy led exercise programme, incorporating stretching, strengthening, physical activity, and behavioural change techniques to support adherence to exercise, introduced at 7-10 days postoperatively, with two further appointments at one and three months.
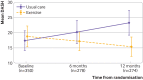
Main outcome measures: Disability of Arm, Hand and Shoulder (DASH) questionnaire at 12 months, analysed by intention to treat. Secondary outcomes included DASH subscales, pain, complications, health related quality of life, and resource use, from a health and personal social services perspective.
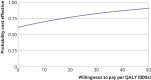
Results: Between 26 January 2016 and 31 July 2017, 951 patients were screened and 392 (mean age 58.1 years) were randomly allocated, with 382 (97%) eligible for intention to treat analysis. 181 (95%) of 191 participants allocated to exercise attended at least one appointment. Upper limb function improved after exercise compared with usual care (mean DASH 16.3 (SD 17.6) for exercise (n=132); 23.7 (22.9) usual care (n=138); adjusted mean difference 7.81, 95% confidence interval 3.17 to 12.44; P=0.001). Secondary outcomes favoured exercise over usual care, with lower pain intensity at 12 months (adjusted mean difference on numerical rating scale -0.68, -1.23 to -0.12; P=0.02) and fewer arm disability symptoms at 12 months (adjusted mean difference on Functional Assessment of Cancer Therapy-Breast+4 (FACT-B+4) -2.02, -3.11 to -0.93; P=0.001). No increase in complications, lymphoedema, or adverse events was noted in participants allocated to exercise. Exercise accrued lower costs per patient (on average -£387 (€457; $533) (95% confidence interval -£2491 to £1718; 2015 pricing) and was cost effective compared with usual care.
Conclusions: The PROSPER exercise programme was clinically effective and cost effective and reduced upper limb disability one year after breast cancer treatment in patients at risk of treatment related postoperative complications.
Trial registration: ISRCTN Registry ISRCTN35358984.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the UK National Institute for Health Research (NIHR) Technology Assessment Programme; JB received grants from the UK NIHR during the conduct of this study and is a member of the NIHR Research for Patient Benefit board; SL reports membership of the UK NIHR Health Technology Assessment (HTA) Additional Capacity Funding Board, HTA End of Life Care and Add-on Studies Board, HTA Prioritisation Group Board, and HTA Trauma Board; no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Critically appraised paper: Exercise is safe, clinically effective and cost-effective compared to usual care after non-reconstructive breast cancer surgery.J Physiother. 2022 Apr;68(2):145. doi: 10.1016/j.jphys.2022.03.004. Epub 2022 Apr 5. J Physiother. 2022. PMID: 35396176 No abstract available.
References
-
- National Institute for Health and Care Excellence. Early and locally advanced breast cancer: diagnosis and management. 2018. https://www.nice.org.uk/guidance/ng101. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
