Clinical development times for innovative drugs
- PMID: 34759309
- PMCID: PMC9869766
- DOI: 10.1038/d41573-021-00190-9
Clinical development times for innovative drugs
Abstract
New drug development is a race against the clock as soon as the first patents are filed, and understanding the potential timings from first-in-human studies to regulatory approval is crucial for strategic planning. Here, we use information gathered from US FDA review documents to provide insight into the timeframes of successful drug development programmes in the past decade. We define clinical development time as the number of days between the initiation of first-in-human clinical studies and regulatory marketing authorization, and we focus on the development of innovative drugs — those products that are being marketed for the first time that contain a new molecular entity or new active moiety (see Supplementary Box 1 for details of the dataset and analysis, and Supplementary Table 1 for the dataset). We also investigate how FDA regulatory programmes may affect this process.
Conflict of interest statement
Competing interests
D.G.B. and H.J.W. are employees of Jnana Therapeutics, which does not have a direct bearing on the subject of this paper. H.J.W is a shareholder in AstraZeneca. The other authors declare no competing interests.
Figures

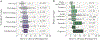
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

