Design of a chitosan-based nano vaccine against epsilon toxin of Clostridium perfringens type D and evaluation of its immunogenicity in BALB/c mice
- PMID: 34760006
- PMCID: PMC8562408
- DOI: 10.4103/1735-5362.327504
Design of a chitosan-based nano vaccine against epsilon toxin of Clostridium perfringens type D and evaluation of its immunogenicity in BALB/c mice
Abstract
Background and purpose: Clostridium perfringens is an anaerobic, spore-forming, and pathogenic bacterium that causes intestinal diseases in humans and animals. In these cases, therapeutic intervention is challenging; because the disease progresses much rapidly. This bacterium can produce 5 main toxins (alpha, beta, epsilon, iota, and a type of enterotoxin) among which the epsilon toxin (ETX) is used for bioterrorism. This toxin can be prevented by immunization with specific immunogenic vaccines. In the present research, we aimed at developing a recombinant chitosan-based nano-vaccine against ETX of C. perfringens and evaluate its effects on the antibody titration against epsilon toxin in BALB/c mice as the vaccine model.
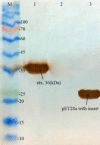
Experimental approach: The etx gene from C. perfringens type D was cloned and expressed in E. coli. After analysis by SDS-PAGE and western blotting, the expressed products were purified, and the obtained proteins were used for immunization in mice as a chitosan nanoparticle containing recombinant, purified ETX, and protein.
Findings/results: The results of ELISA showed that IgA antibody serum level increased sufficiently using recombinant protein with nanoparticle as an oral and injectable formulation. IgG antibody titers increased significantly after administrating the recombinant proteins with nanoparticles through both oral delivery and intravenous injection.
Conclusion and implication: In conclusion, the recombinant ETX is suggested as a good candidate for vaccine production against diseases caused by ETX of C. perfringens type D.
Keywords: Chitosan; Clostridium perfringens; Epsilon toxin; Immunization; Nano-vaccine.
Copyright: © 2021 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Figures






References
-
- Hassan KA, Elbourne LD, Tetu SG, Melville SB, Rood JI, Paulsen IT. Genomic analyses of Clostridium perfringens isolates from five toxinotypes. Res Microbiol. 2015;166(4):255–263. DOI: 10.1016/j.resmic.2014.10.003. - PubMed
-
- Heida FH, van Zoonen AG, Hulscher JB, Te Kiefte BJ, Wessels R, Kooi EM, et al. A necrotizing enterocolitis-associated gut microbiota is present in the meconium: results of a prospective study. Clin Infect Dis. 2016;62(7):863–870. DOI: 10.1093/cid/ciw016. - PubMed
-
- Titball RW. Clostridium perfringens vaccines. Vaccine. 2009;27(Suppl 4):D44–D477. DOI: 10.1016/j.vaccine.2009.07.047. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
