Artemether-loaded nanostructured lipid carriers: preparation, characterization, and evaluation of in vitro effect on Leishmania major
- PMID: 34760010
- PMCID: PMC8562414
- DOI: 10.4103/1735-5362.327508
Artemether-loaded nanostructured lipid carriers: preparation, characterization, and evaluation of in vitro effect on Leishmania major
Abstract
Background and purpose: Cutaneous leishmaniasis is a global health problem. The discovery of new and highly efficient anti-leishmanial treatments with lower toxicity is globally needed. The current study was carried out to evaluate the anti-leishmanial effects of artemether (ART) and ART-loaded nanostructured lipid carriers (ART-NLCs) against promastigotes and amastigotes of Leishmania major.
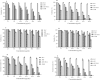
Experimental approach: Solvent diffusion evaporation technique was applied to prepare ART-NLCs. These nanoparticles were characterized using a particle size analyzer (PSA), transmission electron microscopy (TEM), and dynamic light scattering (DLS). The antiparasitic activity on amastigote was assessed in J774 cell culture. The drug cytotoxicity on promastigote and macrophage was assessed using the MTT technique after 24 and 48 h and compared with NLCs, ART, and amphotericin B, as the control agents. The selectivity index was calculated for the agents.
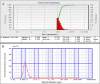
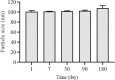
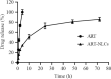
Findings/results: The DLS and PSA techniques confirmed that ART-NLCs were homogenous in size with an average diameter of 101 ± 2.0 nm and span index of 0.9. The ART-NLCs significantly heighten the anti-leishmanial activity of ART (P < 0.001). The IC50 values of ART and ART-NLCs on promastigotes after 24 and 48 h were 76.08, 36.71 and 35.14, 14.81 μg/mL, respectively while they were calculated 53.97, 25.43 and 20.13, 11.92 for amastigotes. Also, ART-NLCs had the lowest cytotoxicity against macrophages. Furthermore, among the agents tested, ART-NLCs had the highest selectivity index.
Conclusion and implications: ART-NLCs had lower cytotoxic effects than ART and amphotericin B, also its selectivity index was significantly higher. Based on the findings of the study, this formulation could be a promising candidate for further research into leishmaniasis treatment.
Keywords: Artemether; L. major; Leishmaniasis; Nanostructured lipid carriers.
Copyright: © 2021 Research in Pharmaceutical Sciences.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Figures


Similar articles
-
Preparation and characterization of artemether-loaded niosomes in Leishmania major-induced cutaneous leishmaniasis.Sci Rep. 2024 May 2;14(1):10073. doi: 10.1038/s41598-024-60883-0. Sci Rep. 2024. PMID: 38698123 Free PMC article.
-
Development, in vitro and in vivo evaluation of miltefosine loaded nanostructured lipid carriers for the treatment of Cutaneous Leishmaniasis.Int J Pharm. 2021 Jan 25;593:120109. doi: 10.1016/j.ijpharm.2020.120109. Epub 2020 Nov 28. Int J Pharm. 2021. PMID: 33253802
-
In vitro effect of artemether-loaded nanostructured lipid carrier (NLC) on Leishmania infantum.J Parasit Dis. 2021 Dec;45(4):964-971. doi: 10.1007/s12639-021-01373-2. Epub 2021 Apr 16. J Parasit Dis. 2021. PMID: 34789979 Free PMC article.
-
Encapsulation of Citrus sinensis essential oil and R-limonene in lipid nanocarriers: A potential strategy for the treatment of leishmaniasis.Int J Pharm. 2024 Sep 5;662:124464. doi: 10.1016/j.ijpharm.2024.124464. Epub 2024 Jul 19. Int J Pharm. 2024. PMID: 39033939
-
Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment.Eur J Pharm Sci. 2020 Jul 1;150:105335. doi: 10.1016/j.ejps.2020.105335. Epub 2020 Apr 6. Eur J Pharm Sci. 2020. PMID: 32272211
Cited by
-
Zinc selenide (ZnSe) nanoparticle coated with green seaweed (Ulva fasciata) hydroalcoholic extract as an anti-leishmanial compound on Leishmania major.PLoS One. 2025 Apr 29;20(4):e0321219. doi: 10.1371/journal.pone.0321219. eCollection 2025. PLoS One. 2025. PMID: 40299894 Free PMC article.
-
Preparation and characterization of artemether-loaded niosomes in Leishmania major-induced cutaneous leishmaniasis.Sci Rep. 2024 May 2;14(1):10073. doi: 10.1038/s41598-024-60883-0. Sci Rep. 2024. PMID: 38698123 Free PMC article.
-
Immunoinformatic approach to the design of a novel multi-epitope vaccine against Leishmania major fused to human IgG-Fc.Res Pharm Sci. 2024 Dec 15;19(6):729-745. doi: 10.4103/RPS.RPS_145_24. eCollection 2024 Dec. Res Pharm Sci. 2024. PMID: 39911897 Free PMC article.
-
Curcumin-loaded nanostructured systems for treatment of leishmaniasis: a review.Beilstein J Nanotechnol. 2024 Jan 4;15:37-50. doi: 10.3762/bjnano.15.4. eCollection 2024. Beilstein J Nanotechnol. 2024. PMID: 38213574 Free PMC article. Review.
-
Synthesis and evaluation of nitrochromene derivatives as potential antileishmanial therapeutics through biological and computational studies.Sci Rep. 2025 Jan 20;15(1):2571. doi: 10.1038/s41598-025-86035-6. Sci Rep. 2025. PMID: 39833336 Free PMC article.
References
-
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–970. DOI: 10.1016/S0140-6736(18)31204-2. - PubMed
-
- Gradoni L. Bruschi F, Gradoni L, editors. A Brief Introduction to Leishmaniasis Epidemiology. The Leishmaniases: Old Neglected Tropical Diseases: Springer. 2018:1–13. DOI: 10.1007/978-3-319-72386-0_1.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
