Visible-light-mediated copper photocatalysis for organic syntheses
- PMID: 34760022
- PMCID: PMC8551910
- DOI: 10.3762/bjoc.17.169
Visible-light-mediated copper photocatalysis for organic syntheses
Abstract
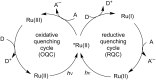
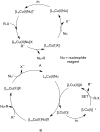
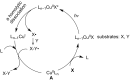
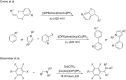
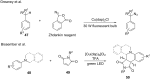
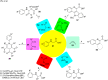
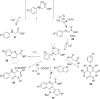
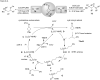
Photoredox catalysis has been applied to renewable energy and green chemistry for many years. Ruthenium and iridium, which can be used as photoredox catalysts, are expensive and scarce in nature. Thus, the further development of catalysts based on these transition metals is discouraged. Alternative photocatalysts based on copper complexes are widely investigated, because they are abundant and less expensive. This review discusses the scope and application of photoinduced copper-based catalysis along with recent progress in this field. The special features and mechanisms of copper photocatalysis and highlights of the applications of the copper complexes to photocatalysis are reported. Copper-photocatalyzed reactions, including alkene and alkyne functionalization, organic halide functionalization, and alkyl C-H functionalization that have been reported over the past 5 years, are included.
Keywords: copper-photocatalyzed reactions; green chemistry; mechanisms of copper photocatalysis; photoinduced copper-based catalysis; photoredox catalysis; special features of copper photocatalysis.
Copyright © 2021, Zhang et al.
Figures
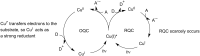



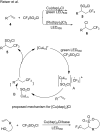



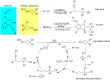



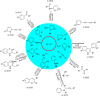

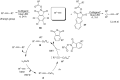







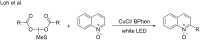
References
-
- Meyer T J. Acc Chem Res. 1989;22:163–170. doi: 10.1021/ar00161a001. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
