Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions
- PMID: 34760176
- PMCID: PMC8549800
- DOI: 10.1039/d1sc04082k
Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions
Abstract
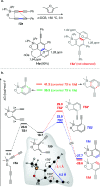
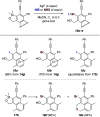
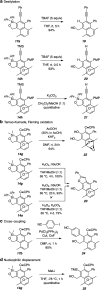
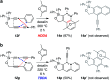
The 1,3-diyne and diynophile in hexadehydro-Diels-Alder (HDDA) reaction substrates are typically tethered by linker units that consist of C, O, N, and/or S atoms. We describe here a new class of polyynes based on silicon-containing tethers that can be disposed of and/or functionalized subsequent to the HDDA reaction. The cyclizations are efficient, and the resulting benzoxasiloles are amenable to protodesilylation, halogenation, oxygenation, and arylation reactions. The presence of the silicon atom can also override the innate mode of cyclization in some cases, an outcome attributable to a β-silyl effect on the structure of intermediate diradicals. Overall, this strategy equates formally to an otherwise unknown, bimolecular HDDA reaction and expands the versatility of this body of aryne chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Rates of hexadehydro-Diels-Alder (HDDA) cyclizations: impact of the linker structure.Org Lett. 2014 Sep 5;16(17):4578-81. doi: 10.1021/ol502131r. Epub 2014 Aug 25. Org Lett. 2014. PMID: 25153729 Free PMC article.
-
Mechanistic and Kinetic Factors of ortho-Benzyne Formation in Hexadehydro-Diels-Alder (HDDA) Reactions.Chemistry. 2021 May 26;27(30):7978-7991. doi: 10.1002/chem.202100608. Epub 2021 May 2. Chemistry. 2021. PMID: 33783896 Free PMC article. Review.
-
Rapid (≤25 °C) cycloisomerization of anhydride-tethered triynes to benzynes - origin of a remarkable anhydride linker-induced rate enhancement.Chem Sci. 2025 Jan 7;16(6):2898-2906. doi: 10.1039/d4sc07232d. eCollection 2025 Feb 5. Chem Sci. 2025. PMID: 39822903 Free PMC article.
-
Quaternary Ammonium Ion-Tethered (Ambient-Temperature) HDDA Reactions.J Am Chem Soc. 2022 May 4;144(17):7750-7757. doi: 10.1021/jacs.2c00877. Epub 2022 Apr 20. J Am Chem Soc. 2022. PMID: 35442671 Free PMC article.
-
Hexadehydro-Diels-Alder Reaction: Benzyne Generation via Cycloisomerization of Tethered Triynes.Chem Rev. 2021 Feb 24;121(4):2413-2444. doi: 10.1021/acs.chemrev.0c00825. Epub 2021 Jan 25. Chem Rev. 2021. PMID: 33492939 Free PMC article. Review.
Cited by
-
An Untethered and Formal Intermolecular Hexadehydro-Diels-Alder Reaction: Alkynylboronates with 2-(1,3-Butadiynyl)pyridines.J Am Chem Soc. 2024 Dec 18;146(50):34510-34516. doi: 10.1021/jacs.4c11622. Epub 2024 Dec 6. J Am Chem Soc. 2024. PMID: 39641921
-
Acid-catalyzed, Three-component, Spontaneous Cascades of 1,3-Butadiynyl Propargylic Alcohols as a Route to Phthalan Derivatives.ACS Catal. 2025 Jul 18;15(14):12238-12246. doi: 10.1021/acscatal.5c03571. Epub 2025 Jul 6. ACS Catal. 2025. PMID: 40703645
-
Utilizing the Pentadehydro-Diels-Alder Reaction for Polycyclic Aromatic Compound Synthesis: Diels-Alder-Based Linker Transformation.Molecules. 2025 Apr 4;30(7):1617. doi: 10.3390/molecules30071617. Molecules. 2025. PMID: 40286250 Free PMC article.
References
-
- Bradley A. Z. Johnson R. P. J. Am. Chem. Soc. 1997;119:9917–9918. doi: 10.1021/ja972141f. - DOI
- Miyawaki K. Suzuki R. Kawano T. Ueda I. Tetrahedron Lett. 1997;38:3943–3946. doi: 10.1016/S0040-4039(97)00785-5. - DOI
- Hoye T. R. Baire B. Niu D. Willoughby P. H. Woods B. P. Nature. 2012;490:208–212. doi: 10.1038/nature11518. - DOI - PMC - PubMed
-
- née Hall C. H. Greaney M. F. Angew. Chem., Int. Ed. 2014;53:5746–5749. doi: 10.1002/anie.201402405. - DOI - PMC - PubMed
- Diamond O. J. Marder T. B. Org. Chem. Front. 2017;4:891–910. doi: 10.1039/C7QO00071E. - DOI
- Fluegel L. L. Hoye T. R. Chem. Rev. 2021;121:2413–2444. doi: 10.1021/acs.chemrev.0c00825. - DOI - PMC - PubMed
-
- Bols M. Skrydstrup T. Chem. Rev. 1995;95:1253–1277. doi: 10.1021/cr00037a006. - DOI
- Fensterbank L. Max M. Sieburth S. Synthesis. 1997;7:813–854. doi: 10.1055/s-1997-1295. - DOI
- Gauthier Jr D. R. Zandi K. S. Shea K. J. Tetrahedron. 1998;54:2289–2338. doi: 10.1016/S0040-4020(97)10304-0. - DOI
- Bracegirdle S. Anderson E. A. Chem. Soc. Rev. 2010;39:4114–4129. doi: 10.1039/C0CS00007H. - DOI - PubMed
- Evans P. A., Temporary Silicon-Tethered Ring-Closing Metathesis Reactions in Natural Product Synthesis, in Metathesis in Natural Product Synthesis, ed. J. Cossy, S. Areniyadis and C. Meyer, Wiley-VCH, Weinheim, Germany, 2010, pp. 225–259
- Parasram M. Gevorgyan V. Acc. Chem. Res. 2017;50:2038–2053. doi: 10.1021/acs.accounts.7b00306. - DOI - PMC - PubMed
- Usman M. Zhang X.-W. Liu W.-B. Synthesis. 2019;51:1529–1544. doi: 10.1055/s-0037-1612123. - DOI
-
- Himeshima Y. Sonoda T. Kobayashi H. Chem. Lett. 1983:1211–1214. doi: 10.1246/cl.1983.1211. - DOI
LinkOut - more resources
Full Text Sources

