Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions
- PMID: 34760176
- PMCID: PMC8549800
- DOI: 10.1039/d1sc04082k
Silicon as a powerful control element in HDDA chemistry: redirection of innate cyclization preferences, functionalizable tethers, and formal bimolecular HDDA reactions
Abstract
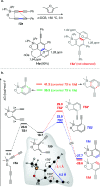
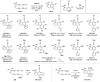
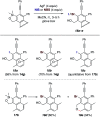
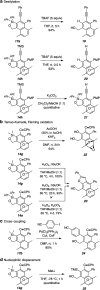
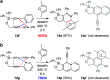
The 1,3-diyne and diynophile in hexadehydro-Diels-Alder (HDDA) reaction substrates are typically tethered by linker units that consist of C, O, N, and/or S atoms. We describe here a new class of polyynes based on silicon-containing tethers that can be disposed of and/or functionalized subsequent to the HDDA reaction. The cyclizations are efficient, and the resulting benzoxasiloles are amenable to protodesilylation, halogenation, oxygenation, and arylation reactions. The presence of the silicon atom can also override the innate mode of cyclization in some cases, an outcome attributable to a β-silyl effect on the structure of intermediate diradicals. Overall, this strategy equates formally to an otherwise unknown, bimolecular HDDA reaction and expands the versatility of this body of aryne chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Bradley A. Z. Johnson R. P. J. Am. Chem. Soc. 1997;119:9917–9918. doi: 10.1021/ja972141f. - DOI
- Miyawaki K. Suzuki R. Kawano T. Ueda I. Tetrahedron Lett. 1997;38:3943–3946. doi: 10.1016/S0040-4039(97)00785-5. - DOI
- Hoye T. R. Baire B. Niu D. Willoughby P. H. Woods B. P. Nature. 2012;490:208–212. doi: 10.1038/nature11518. - DOI - PMC - PubMed
-
- née Hall C. H. Greaney M. F. Angew. Chem., Int. Ed. 2014;53:5746–5749. doi: 10.1002/anie.201402405. - DOI - PMC - PubMed
- Diamond O. J. Marder T. B. Org. Chem. Front. 2017;4:891–910. doi: 10.1039/C7QO00071E. - DOI
- Fluegel L. L. Hoye T. R. Chem. Rev. 2021;121:2413–2444. doi: 10.1021/acs.chemrev.0c00825. - DOI - PMC - PubMed
-
- Bols M. Skrydstrup T. Chem. Rev. 1995;95:1253–1277. doi: 10.1021/cr00037a006. - DOI
- Fensterbank L. Max M. Sieburth S. Synthesis. 1997;7:813–854. doi: 10.1055/s-1997-1295. - DOI
- Gauthier Jr D. R. Zandi K. S. Shea K. J. Tetrahedron. 1998;54:2289–2338. doi: 10.1016/S0040-4020(97)10304-0. - DOI
- Bracegirdle S. Anderson E. A. Chem. Soc. Rev. 2010;39:4114–4129. doi: 10.1039/C0CS00007H. - DOI - PubMed
- Evans P. A., Temporary Silicon-Tethered Ring-Closing Metathesis Reactions in Natural Product Synthesis, in Metathesis in Natural Product Synthesis, ed. J. Cossy, S. Areniyadis and C. Meyer, Wiley-VCH, Weinheim, Germany, 2010, pp. 225–259
- Parasram M. Gevorgyan V. Acc. Chem. Res. 2017;50:2038–2053. doi: 10.1021/acs.accounts.7b00306. - DOI - PMC - PubMed
- Usman M. Zhang X.-W. Liu W.-B. Synthesis. 2019;51:1529–1544. doi: 10.1055/s-0037-1612123. - DOI
-
- Himeshima Y. Sonoda T. Kobayashi H. Chem. Lett. 1983:1211–1214. doi: 10.1246/cl.1983.1211. - DOI
LinkOut - more resources
Full Text Sources

