Lipid-mimicking phosphorus-based glycosidase inactivators as pharmacological chaperones for the treatment of Gaucher's disease
- PMID: 34760177
- PMCID: PMC8549773
- DOI: 10.1039/d1sc03831a
Lipid-mimicking phosphorus-based glycosidase inactivators as pharmacological chaperones for the treatment of Gaucher's disease
Abstract
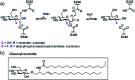
Gaucher's disease, the most prevalent lysosomal storage disorder, is caused by missense mutation of the GBA gene, ultimately resulting in deficient GCase activity, hence the excessive build-up of cellular glucosylceramide. Among different therapeutic strategies, pharmacological chaperoning of mutant GCase represents an attractive approach that relies on small organic molecules acting as protein stabilizers. Herein, we expand upon a new class of transient GCase inactivators based on a reactive 2-deoxy-2-fluoro-β-d-glucoside tethered to an array of lipid-mimicking phosphorus-based aglycones, which not only improve the selectivity and inactivation efficiency, but also the stability of these compounds in aqueous media. This hypothesis was further validated with kinetic and cellular studies confirming restoration of catalytic activity in Gaucher cells after treatment with these pharmacological chaperones.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Glucocerebrosidase Mutations Cause Mitochondrial and Lysosomal Dysfunction in Parkinson's Disease: Pathogenesis and Therapeutic Implications.Front Aging Neurosci. 2022 Mar 23;14:851135. doi: 10.3389/fnagi.2022.851135. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35401150 Free PMC article. Review.
-
Pharmacological Chaperones for GCase that Switch Conformation with pH Enhance Enzyme Levels in Gaucher Animal Models.Angew Chem Int Ed Engl. 2022 Sep 19;61(38):e202207974. doi: 10.1002/anie.202207974. Epub 2022 Aug 12. Angew Chem Int Ed Engl. 2022. PMID: 35864061
-
Glucosylceramide mimics: highly potent GCase inhibitors and selective pharmacological chaperones for mutations associated with types 1 and 2 Gaucher disease.ChemMedChem. 2013 Nov;8(11):1805-17. doi: 10.1002/cmdc.201300327. Epub 2013 Oct 2. ChemMedChem. 2013. PMID: 24115322
-
Measurement of GCase Activity in Cultured Cells.Methods Mol Biol. 2021;2322:47-52. doi: 10.1007/978-1-0716-1495-2_5. Methods Mol Biol. 2021. PMID: 34043191
-
Cross-talks among GBA mutations, glucocerebrosidase, and α-synuclein in GBA-associated Parkinson's disease and their targeted therapeutic approaches: a comprehensive review.Transl Neurodegener. 2021 Jan 15;10(1):4. doi: 10.1186/s40035-020-00226-x. Transl Neurodegener. 2021. PMID: 33446243 Free PMC article. Review.
Cited by
-
Promising therapeutic aspects in human genetic imprinting disorders.Clin Epigenetics. 2022 Nov 12;14(1):146. doi: 10.1186/s13148-022-01369-6. Clin Epigenetics. 2022. PMID: 36371218 Free PMC article. Review.
-
Development of Tunable Mechanism-Based Carbasugar Ligands that Stabilize Glycoside Hydrolases through the Formation of Transient Covalent Intermediates.ACS Catal. 2024 Sep 20;14(19):14769-14779. doi: 10.1021/acscatal.4c04549. eCollection 2024 Oct 4. ACS Catal. 2024. PMID: 39386917 Free PMC article.
-
Mechanistic Insights into Dibasic Iminosugars as pH-Selective Pharmacological Chaperones to Stabilize Human α-Galactosidase.JACS Au. 2024 Feb 23;4(3):908-918. doi: 10.1021/jacsau.3c00684. eCollection 2024 Mar 25. JACS Au. 2024. PMID: 38559739 Free PMC article.
-
Fluorine-Directed Automated Mannoside Assembly.Angew Chem Int Ed Engl. 2023 Jan 16;62(3):e202213304. doi: 10.1002/anie.202213304. Epub 2022 Dec 12. Angew Chem Int Ed Engl. 2023. PMID: 36331042 Free PMC article.
References
-
- Atashrazm F. Hammond D. Perera G. Dobson-Stone C. Mueller N. Pickford R. Scott Kim W. Kwok J. B. Lewis S. J. G. Halliday G. M. Dzamko N. Sci. Rep. 2018;8:15446. doi: 10.1038/s41598-018-33921-x. - DOI - PMC - PubMed
- Horowitz M. Elstein D. Zimran A. Goker-Alpan O. Hum. Mutat. 2016;37:1121. doi: 10.1002/humu.23056. - DOI - PubMed
LinkOut - more resources
Full Text Sources

