Synthesis, structure and bonding nature of heavy dipnictene radical anions
- PMID: 34760185
- PMCID: PMC8565390
- DOI: 10.1039/d1sc04230k
Synthesis, structure and bonding nature of heavy dipnictene radical anions
Abstract
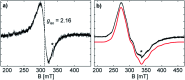
Cyclic voltammetry (CV) studies of two L(X)Ga-substituted dipnictenes [L(R2N)GaE]2 (E = Sb, R = Me 1; E = Bi; R = Et 2; L = HC[C(Me)NDipp]2; Dipp = 2,6-i-Pr2C6H3) showed reversible reduction events. Single electron reduction of 1 and 2 with KC8 in DME in the presence of benzo-18-crown-6 (B-18-C-6) gave the corresponding dipnictenyl radical anions (DME)[K(B-18-C-6)][L(R2N)GaE]2 (E = Sb, R = Me 3; E = Bi, R = Et 4). Radical anions 3 and 4 were characterized by EPR, UV-vis and single crystal X-ray diffraction, while quantum chemical calculations gave deeper insight into the nature of the chemical bonding.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures








References
-
- Yoshifuji M. Shima I. Inamoto N. Hirotsu K. Higuchi T. J. Am. Chem. Soc. 1981;103:4587–4589. doi: 10.1021/ja00405a054. - DOI
-
- Sharma M. K. Blomeyer S. Neumann B. Stammler H. G. Ghadwal R. S. Chem.–Eur. J. 2019;25:8249–8253. doi: 10.1002/chem.201901857. - DOI - PubMed
- Majhi P. K. Ikeda H. Sasamori T. Tsurugi H. Mashima K. Tokitoh N. Organometallics. 2017;36:1224–1226. doi: 10.1021/acs.organomet.7b00147. - DOI
- Sasamori T. Tokitoh N. Dalton Trans. 2008:1395–1408. doi: 10.1039/B715033D. - DOI - PubMed
- Sasamori T. Arai Y. Takeda N. Okazaki R. Furukawa Y. Kimura M. Nagase S. Tokitoh N. Bull. Chem. Soc. Jpn. 2002;75:661–675. doi: 10.1246/bcsj.75.661. - DOI
- Twamley B. Sofield C. D. Olmstead M. M. Power P. P. J. Am. Chem. Soc. 1999;121:3357–3367. doi: 10.1021/ja983999n. - DOI
- Tokitoh N. Arai Y. Sasamori T. Okazaki R. Nagase S. Uekusa H. Ohashi Y. J. Am. Chem. Soc. 1998;120:433–434. doi: 10.1021/ja973295y. - DOI
- Tokitoh N. Arai Y. Okazaki R. Nagase S. Science. 1997;277:78–80. doi: 10.1126/science.277.5322.78. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

