Inhibition of (dppf)nickel-catalysed Suzuki-Miyaura cross-coupling reactions by α-halo-N-heterocycles
- PMID: 34760191
- PMCID: PMC8565371
- DOI: 10.1039/d1sc04582b
Inhibition of (dppf)nickel-catalysed Suzuki-Miyaura cross-coupling reactions by α-halo-N-heterocycles
Abstract
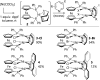
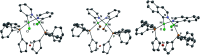
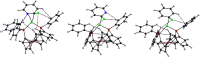
A nickel/dppf catalyst system was found to successfully achieve the Suzuki-Miyaura cross-coupling reactions of 3- and 4-chloropyridine and of 6-chloroquinoline but not of 2-chloropyridine or of other α-halo-N-heterocycles. Further investigations revealed that chloropyridines undergo rapid oxidative addition to [Ni(COD)(dppf)] but that α-halo-N-heterocycles lead to the formation of stable dimeric nickel species that are catalytically inactive in Suzuki-Miyaura cross-coupling reactions. However, the corresponding Kumada-Tamao-Corriu reactions all proceed readily, which is attributed to more rapid transmetalation of Grignard reagents.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







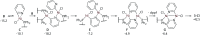
References
LinkOut - more resources
Full Text Sources

