Plasma-assisted oxidation of Cu(100) and Cu(111)
- PMID: 34760210
- PMCID: PMC8565394
- DOI: 10.1039/d1sc04861a
Plasma-assisted oxidation of Cu(100) and Cu(111)
Abstract
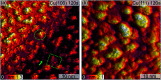
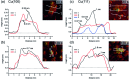
Oxidized copper surfaces have attracted significant attention in recent years due to their unique catalytic properties, including their enhanced hydrocarbon selectivity during the electrochemical reduction of CO2. Although oxygen plasma has been used to create highly active copper oxide electrodes for CO2RR, how such treatment alters the copper surface is still poorly understood. Here, we study the oxidation of Cu(100) and Cu(111) surfaces by sequential exposure to a low-pressure oxygen plasma at room temperature. We used scanning tunnelling microscopy (STM), low energy electron microscopy (LEEM), X-ray photoelectron spectroscopy (XPS), near edge X-ray absorption fine structure spectroscopy (NEXAFS) and low energy electron diffraction (LEED) for the comprehensive characterization of the resulting oxide films. O2-plasma exposure initially induces the growth of 3-dimensional oxide islands surrounded by an O-covered Cu surface. With ongoing plasma exposure, the islands coalesce and form a closed oxide film. Utilizing spectroscopy, we traced the evolution of metallic Cu, Cu2O and CuO species upon oxygen plasma exposure and found a dependence of the surface structure and chemical state on the substrate's orientation. On Cu(100) the oxide islands grow with a lower rate than on the (111) surface. Furthermore, while on Cu(100) only Cu2O is formed during the initial growth phase, both Cu2O and CuO species are simultaneously generated on Cu(111). Finally, prolonged oxygen plasma exposure results in a sandwiched film structure with CuO at the surface and Cu2O at the interface to the metallic support. A stable CuO(111) surface orientation is identified in both cases, aligned to the Cu(111) support, but with two coexisting rotational domains on Cu(100). These findings illustrate the possibility of tailoring the oxidation state, structure and morphology of metallic surfaces for a wide range of applications through oxygen plasma treatments.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Mittiga A. Salza E. Sarto F. Tucci M. Vasanthi R. Appl. Phys. Lett. 2006;88:163502. doi: 10.1063/1.2194315. - DOI
-
- Olsen L. C. Addis F. W. Miller W. Sol. Cells. 1982;7:247–279. doi: 10.1016/0379-6787(82)90050-3. - DOI
-
- Reddy N. L. Emin S. Kumari V. D. Muthukonda Venkatakrishnan S. Ind. Eng. Chem. Res. 2018;57:568–577. doi: 10.1021/acs.iecr.7b03785. - DOI
-
- Zhang Y. Deng B. Zhang T. Gao D. Xu A.-W. J. Phys. Chem. C. 2010;114:5073–5079. doi: 10.1021/jp9110037. - DOI
-
- Hori Y. Wakebe H. Tsukamoto T. Koga O. Electrochim. Acta. 1994;39:1833–1839. doi: 10.1016/0013-4686(94)85172-7. - DOI
LinkOut - more resources
Full Text Sources

