A Role of CXCL1 Drives Osteosarcoma Lung Metastasis via VCAM-1 Production
- PMID: 34760697
- PMCID: PMC8573405
- DOI: 10.3389/fonc.2021.735277
A Role of CXCL1 Drives Osteosarcoma Lung Metastasis via VCAM-1 Production
Abstract
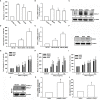
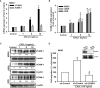
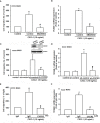
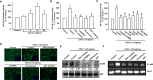
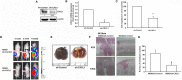
Osteosarcoma, a common aggressive and malignant cancer, appears in the musculoskeletal system among young adults. The major cause of mortality in osteosarcoma was the recurrence of lung metastases. However, the molecular mechanisms of metastasis involved in osteosarcomas remain unclear. Recently, CXCL1 and CXCR2 have been crucial indicators for lung metastasis in osteosarcoma by paracrine releases, suggesting the involvement of directing neutrophils into tumor microenvironment. In this study, overexpression of CXCL1 has a positive correlation with the migratory and invasive activities in osteosarcoma cell lines. Furthermore, the signaling pathway, CXCR2/FAK/PI3K/Akt, is activated through CXCL1 by promoting vascular cell adhesion molecule 1 (VCAM-1) via upregulation of nuclear factor-kappa B (NF-κB) expression and nuclear translocation. The in vivo animal model further demonstrated that CXCL1 serves as a critical promoter in osteosarcoma metastasis to the lung. The correlated expression of CXCL1 and VCAM-1 was observed in the immunohistochemistry staining from human osteosarcoma specimens. Our findings demonstrate the cascade mechanism regulating the network in lung metastasis osteosarcoma, therefore indicating that the CXCL1/CXCR2 pathway is a worthwhile candidate to further develop treatment schemas.
Keywords: CXCL1; VCAM-1; metastasis; migration; osteosarcoma.
Copyright © 2021 Lee, Chiang, Yu, Peng, Chi, Lee, Fang, Lee, Hsu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

