Piscirickettsia salmonis Produces a N-Acetyl-L-Homoserine Lactone as a Bacterial Quorum Sensing System-Related Molecule
- PMID: 34760722
- PMCID: PMC8573184
- DOI: 10.3389/fcimb.2021.755496
Piscirickettsia salmonis Produces a N-Acetyl-L-Homoserine Lactone as a Bacterial Quorum Sensing System-Related Molecule
Abstract
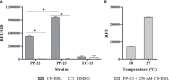
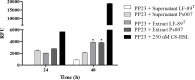
Piscirickettsia salmonis is the etiological agent of piscirickettsiosis, the most prevalent disease in salmonid species in Chilean salmonids farms. Many bacteria produce N-acyl-homoserine lactones (AHLs) as a quorum-sensing signal molecule to regulate gene expression in a cell density-dependent manner, and thus modulate physiological characteristics and several bacterial mechanisms. In this study, a fluorescent biosensor system method and gas chromatography-tandem mass spectrometry (GC/MS) were combined to detect AHLs produced by P. salmonis. These analyses revealed an emitted fluorescence signal when the biosensor P. putida EL106 (RPL4cep) was co-cultured with both, P. salmonis LF-89 type strain and an EM-90-like strain Ps007, respectively. Furthermore, the production of an AHL-type molecule was confirmed by GC/MS by both P. salmonis strains, which identified the presence of a N-acetyl-L-homoserine Lactone in the supernatant extract. However, It is suggested that an alternate pathway could synthesizes AHLs, which should be address in future experiments in order to elucidate this important bacterial process. To the best of our knowledge, the present report is the first to describe the type of AHLs produced by P. salmonis.
Keywords: AHL (N-acyl-homoserine lactone); N-acetyl-L-homoserine lactone; Piscirickettsia salmonis; SRS; quorum sensing (QS).
Copyright © 2021 Ruiz, Sepulveda, Vidal, Romero, Contreras, Barros, Carrasco, Ruiz-Tagle, Romero, Urrutia and Oliver.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Comment in
-
Commentary: Piscirickettsia salmonis Produces a N-Acetyl-L-Homoserine Lactone as a Bacterial Quorum Sensing System-Related Molecule.Front Cell Infect Microbiol. 2022 Mar 4;12:858387. doi: 10.3389/fcimb.2022.858387. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35310837 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

