Agreement between WHO-UMC causality scale and the Naranjo algorithm for causality assessment of adverse drug reactions
- PMID: 34760748
- PMCID: PMC8565125
- DOI: 10.4103/jfmpc.jfmpc_831_21
Agreement between WHO-UMC causality scale and the Naranjo algorithm for causality assessment of adverse drug reactions
Abstract
Background: The Pharmacovigilance Program of India recommends the use of the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) scale, while many clinicians prefer the Naranjo algorithm for its simplicity. In the present study, we assessed agreement between the two widely used causality assessment scales, that is, the WHO-UMC criteria and the Naranjo algorithm.
Materials and methods: In this study, 842 individual case safety reports were randomly selected from 1000 spontaneously reported forms submitted to the ADR Monitoring Center at a tertiary healthcare Institute in Central India between 2016 and 2018. Two well-trained independent groups performed the causality assessment. One group performed a causality assessment of the 842 ADRs using the WHO-UMC criteria and the other group performed the same using the Naranjo algorithm. The agreement between two ADR causality scales was assessed using the weighted kappa (κ) test.
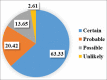
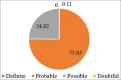
Results: Cohen's kappa coefficient (κ) statistical test was applied between the two scales (WHO-UMC scale and Naranjo algorithm) to find out the agreement between these two scales. "No" agreement was found between the two scales {Kappa statistic with 95% confidence interval = 0.048 (P < 0.001)}.
Conclusion: There was no agreement found between the WHO-UMC criteria and the Naranjo algorithm in our study.
Keywords: Agreement between scales; Naranjo algorithm; WHO-UMC scale; causality assessment.
Copyright: © 2021 Journal of Family Medicine and Primary Care.
Conflict of interest statement
There are no conflicts of interest.
Figures
Comment in
-
Drug-induced glove and stocking distribution rash: a rare entity in the intensive care unit.Int J Pharm Pract. 2022 Jun 25;30(3):292-293. doi: 10.1093/ijpp/riac018. Int J Pharm Pract. 2022. PMID: 35262673 No abstract available.
References
-
- Mulchandani R, Kakkar AK. Reporting of adverse drug reactions in India: A review of the current scenario, obstacles and possible solutions. Int J Risk Saf Med. 2019;30:33–44. - PubMed
-
- World Health Organization. Geneva: World Health Organization; 2002. Safety of medicines. A guide to detecting and reporting adverse drug reactions – why health professionals need to take action.
-
- Agbabiaka TB, Savovicx J, Ernst E. Methods for causality assessment of adverse drug reactions: A systematic review. Drug Saf. 2008;31:21–37. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials