Normal limits for oscillometric bronchodilator responses and relationships with clinical factors
- PMID: 34761000
- PMCID: PMC8573235
- DOI: 10.1183/23120541.00439-2021
Normal limits for oscillometric bronchodilator responses and relationships with clinical factors
Abstract
Introduction: We aimed to determine normal thresholds for positive bronchodilator responses for oscillometry in an Australian general population sample aged ≥40 years, to guide clinical interpretation. We also examined relationships between bronchodilator responses and respiratory symptoms, asthma diagnosis, smoking and baseline lung function.
Methods: Subjects recruited from Sydney, Melbourne and Busselton, Australia, underwent measurements of spirometry, resistance (R rs6 ) and reactance (X rs6 ) at 6 Hz, before and after inhalation of salbutamol 200 μg. Respiratory symptoms and/or medication use, asthma diagnosis, and smoking were recorded. Threshold bronchodilator responses were defined as the fifth percentile of decrease in R rs6 and 95th percentile increase in X rs6 in a healthy subgroup.
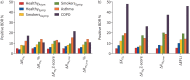
Results: Of 1318 participants, 1145 (570 female) were analysed. The lower threshold for ΔR rs6 was -1.38 cmH2O·s·L-1 (-30.0% or -1.42 Z-scores) and upper threshold for ΔX rs6 was 0.57 cmH2O·s·L-1 (1.36 Z-scores). Respiratory symptoms and/or medication use, asthma diagnosis, and smoking all predicted bronchodilator response, as did baseline oscillometry and spirometry. When categorised into clinically relevant groups according to those predictors, ΔX rs6 was more sensitive than spirometry in smokers without current asthma or chronic obstructive pulmonary disease (COPD), ∼20% having a positive response. Using absolute or Z-score change provided similar prevalences of responsiveness, except in COPD, in which responsiveness measured by absolute change was twice that for Z-score.
Discussion: This study describes normative thresholds for bronchodilator responses in oscillometry parameters, including intra-breath parameters, as determined by absolute, relative and Z-score changes. Positive bronchodilator response by oscillometry correlated with clinical factors and baseline function, which may inform the clinical interpretation of oscillometry.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: K. Jetmalani is a part-time employee of GlaxoSmithKline. Conflict of interest: N.J. Brown has nothing to disclose. Conflict of interest: C. Boustany has nothing to disclose. Conflict of interest: B.G. Toelle has nothing to disclose. Conflict of interest: G.B. Marks has nothing to disclose. Conflict of interest: M.J. Abramson reports an investigator-initiated grant to conduct the BOLD study in Australia from the National Health and Medical Research Council during the conduct of the study; and investigator-initiated grants for unrelated research from Pfizer and Boehringer Ingelheim, personal fees for unrelated consultancies and assistance with congress attendance from Sanofi, and a speaker's fee from GlaxoSmithKline, outside the submitted work. Conflict of interest: D.P. Johns has nothing to disclose. Conflict of interest: A.L. James has nothing to disclose. Conflict of interest: M. Hunter has nothing to disclose. Conflict of interest: A.W. Musk has nothing to disclose. Conflict of interest: N. Berend is a part-time employee of GlaxoSmithKline. Conflict of interest: C.S. Farah has nothing to disclose. Conflict of interest: D.G. Chapman has nothing to disclose. Conflict of interest: C. Thamrin has a patent WO 2006130922 A1 issued, which is broadly relevant to the work. In addition, she has intellectual property arrangements with Thorasys, Thoracic Medical Systems and Restech srl relating to research collaborations, but does not have any financial relationships with either company. Conflict of interest: G.G. King reports intellectual property arrangements covering research collaborations and provision of FOT devices for research from Restech during the conduct of the study; fees for consultancy services (which include lectures and advisory board services), conference attendance support and unrestricted research grants from AstraZeneca, Boehringer Ingelheim, CycloPharm, GlaxoSmithKline, Novartis, Menarini and MundiPharma, and research grants and fellowships from the National Health and Medical Research Council, the Asthma Foundation and philanthropic donations via Sydney University, outside the submitted work.
Figures

References
LinkOut - more resources
Full Text Sources
